Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions
- PMID: 20145096
- PMCID: PMC2849392
- DOI: 10.1128/IAI.01171-09
Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions
Abstract
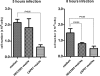
Cryptococcus neoformans and distantly related fungal species release extracellular vesicles that traverse the cell wall and contain a varied assortment of components, some of which have been associated with virulence. Previous studies have suggested that these extracellular vesicles are produced in vitro and during animal infection, but the role of vesicular secretion during the interaction of fungi with host cells remains unknown. In this report, we demonstrate by fluorescence microscopy that mammalian macrophages can incorporate extracellular vesicles produced by C. neoformans. Incubation of cryptococcal vesicles with murine macrophages resulted in increased levels of extracellular tumor necrosis factor alpha (TNF-alpha), interleukin-10 (IL-10), and transforming growth factor beta (TGF-beta). Vesicle preparations also resulted in a dose-dependent stimulation of nitric oxide production by phagocytes, suggesting that vesicle components stimulate macrophages to produce antimicrobial compounds. Treated macrophages were more effective at killing C. neoformans yeast. Our results indicate that the extracellular vesicles of C. neoformans can stimulate macrophage function, apparently activating these phagocytic cells to enhance their antimicrobial activity. These results establish that cryptococcal vesicles are biologically active.
Figures


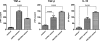

Similar articles
-
Cryptococcus neoformans-Infected Macrophages Release Proinflammatory Extracellular Vesicles: Insight into Their Components by Multi-omics.mBio. 2021 Mar 30;12(2):e00279-21. doi: 10.1128/mBio.00279-21. mBio. 2021. PMID: 33785616 Free PMC article.
-
Cytokine enhancement of complement-dependent phagocytosis by macrophages: synergy of tumor necrosis factor-alpha and granulocyte-macrophage colony-stimulating factor for phagocytosis of Cryptococcus neoformans.Eur J Immunol. 1992 Jun;22(6):1447-54. doi: 10.1002/eji.1830220617. Eur J Immunol. 1992. PMID: 1601035
-
Cryptococcus neoformans fails to induce nitric oxide synthase in primed murine macrophage-like cells.Infect Immun. 1995 Apr;63(4):1298-304. doi: 10.1128/iai.63.4.1298-1304.1995. Infect Immun. 1995. PMID: 7534274 Free PMC article.
-
Pulmonary Macrophage and Dendritic Cell Responses to Cryptococcus neoformans.Front Cell Infect Microbiol. 2020 Feb 11;10:37. doi: 10.3389/fcimb.2020.00037. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32117810 Free PMC article. Review.
-
Catch me if you can: phagocytosis and killing avoidance by Cryptococcus neoformans.FEMS Immunol Med Microbiol. 2012 Mar;64(2):147-61. doi: 10.1111/j.1574-695X.2011.00871.x. FEMS Immunol Med Microbiol. 2012. PMID: 22029633 Review.
Cited by
-
Kicking sleepers out of bed: Macrophages promote reactivation of dormant Cryptococcus neoformans by extracellular vesicle release and non-lytic exocytosis.PLoS Pathog. 2023 Nov 30;19(11):e1011841. doi: 10.1371/journal.ppat.1011841. eCollection 2023 Nov. PLoS Pathog. 2023. PMID: 38033163 Free PMC article.
-
Extracellular Vesicle Formation in Cryptococcus deuterogattii Impacts Fungal Virulence and Requires the NOP16 Gene.Infect Immun. 2022 Aug 18;90(8):e0023222. doi: 10.1128/iai.00232-22. Epub 2022 Jul 12. Infect Immun. 2022. Corrected and republished in: Infect Immun. 2024 Apr 9;92(4):e0003724. doi: 10.1128/iai.00037-24. PMID: 35862719 Free PMC article. Corrected and republished.
-
Lyophilization induces physicochemical alterations in cryptococcal exopolysaccharide.Carbohydr Polym. 2022 Sep 1;291:119547. doi: 10.1016/j.carbpol.2022.119547. Epub 2022 Apr 29. Carbohydr Polym. 2022. PMID: 35698377 Free PMC article.
-
Immunomodulatory Potential of Fungal Extracellular Vesicles: Insights for Therapeutic Applications.Biomolecules. 2023 Oct 6;13(10):1487. doi: 10.3390/biom13101487. Biomolecules. 2023. PMID: 37892168 Free PMC article. Review.
-
Immunoproteomic Approach of Extracellular Antigens From Paracoccidioides Species Reveals Exclusive B-Cell Epitopes.Front Microbiol. 2020 Jan 28;10:2968. doi: 10.3389/fmicb.2019.02968. eCollection 2019. Front Microbiol. 2020. PMID: 32117076 Free PMC article.
References
-
- Albuquerque, P. C., E. S. Nakayasu, M. L. Rodrigues, S. Frases, A. Casadevall, R. M. Zancope-Oliveira, I. C. Almeida, and J. D. Nosanchuk. 2008. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol. 10:1695-1710. - PMC - PubMed
-
- Alvarez, M., and A. Casadevall. 2006. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 16:2161-2165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

