Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency
- PMID: 20145110
- PMCID: PMC2841930
- DOI: 10.1073/pnas.0908790107
Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency
Abstract
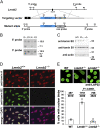
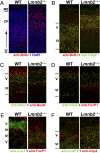
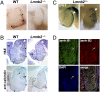
Nuclear lamins are components of the nuclear lamina, a structural scaffolding for the cell nucleus. Defects in lamins A and C cause an array of human diseases, including muscular dystrophy, lipodystrophy, and progeria, but no diseases have been linked to the loss of lamins B1 or B2. To explore the functional relevance of lamin B2, we generated lamin B2-deficient mice and found that they have severe brain abnormalities resembling lissencephaly, with abnormal layering of neurons in the cerebral cortex and cerebellum. This neuronal layering abnormality is due to defective neuronal migration, a process that is dependent on the organized movement of the nucleus within the cell. These studies establish an essential function for lamin B2 in neuronal migration and brain development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Nuclear envelope and lamin B2 function in the central nervous system.Proc Natl Acad Sci U S A. 2010 Apr 6;107(14):6121-2. doi: 10.1073/pnas.1000863107. Epub 2010 Apr 1. Proc Natl Acad Sci U S A. 2010. PMID: 20360557 Free PMC article. No abstract available.
References
-
- Stewart CL, Roux KJ, Burke B. Blurring the boundary: The nuclear envelope extends its reach. Science. 2007;318:1408–1412. - PubMed
-
- Zheng Y, Tsai MY. The mitotic spindle matrix: A fibro-membranous lamin connection. Cell Cycle. 2006;5:2345–2347. - PubMed
-
- Hutchison CJ. Lamins: Building blocks or regulators of gene expression? Nat Rev Mol Cell Biol. 2002;3:848–858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

