Structural determinants of growth factor binding and specificity by VEGF receptor 2
- PMID: 20145116
- PMCID: PMC2823880
- DOI: 10.1073/pnas.0914318107
Structural determinants of growth factor binding and specificity by VEGF receptor 2
Abstract
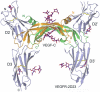
Vascular endothelial growth factors (VEGFs) regulate blood and lymph vessel formation through activation of three receptor tyrosine kinases, VEGFR-1, -2, and -3. The extracellular domain of VEGF receptors consists of seven immunoglobulin homology domains, which, upon ligand binding, promote receptor dimerization. Dimerization initiates transmembrane signaling, which activates the intracellular tyrosine kinase domain of the receptor. VEGF-C stimulates lymphangiogenesis and contributes to pathological angiogenesis via VEGFR-3. However, proteolytically processed VEGF-C also stimulates VEGFR-2, the predominant transducer of signals required for physiological and pathological angiogenesis. Here we present the crystal structure of VEGF-C bound to the VEGFR-2 high-affinity-binding site, which consists of immunoglobulin homology domains D2 and D3. This structure reveals a symmetrical 22 complex, in which left-handed twisted receptor domains wrap around the 2-fold axis of VEGF-C. In the VEGFs, receptor specificity is determined by an N-terminal alpha helix and three peptide loops. Our structure shows that two of these loops in VEGF-C bind to VEGFR-2 subdomains D2 and D3, while one interacts primarily with D3. Additionally, the N-terminal helix of VEGF-C interacts with D2, and the groove separating the two VEGF-C monomers binds to the D2/D3 linker. VEGF-C, unlike VEGF-A, does not bind VEGFR-1. We therefore created VEGFR-1/VEGFR-2 chimeric proteins to further study receptor specificity. This biochemical analysis, together with our structural data, defined VEGFR-2 residues critical for the binding of VEGF-A and VEGF-C. Our results provide significant insights into the structural features that determine the high affinity and specificity of VEGF/VEGFR interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
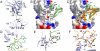

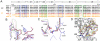
Similar articles
-
Structural and mechanistic insights into VEGF receptor 3 ligand binding and activation.Proc Natl Acad Sci U S A. 2013 Aug 6;110(32):12960-5. doi: 10.1073/pnas.1301415110. Epub 2013 Jul 22. Proc Natl Acad Sci U S A. 2013. PMID: 23878260 Free PMC article.
-
The basis for the distinct biological activities of vascular endothelial growth factor receptor-1 ligands.Sci Signal. 2013 Jul 2;6(282):ra52. doi: 10.1126/scisignal.2003905. Sci Signal. 2013. PMID: 23821770
-
Structural determinants of vascular endothelial growth factor-D receptor binding and specificity.Blood. 2011 Feb 3;117(5):1507-15. doi: 10.1182/blood-2010-08-301549. Epub 2010 Dec 8. Blood. 2011. PMID: 21148085
-
Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases.J Biochem. 2013 Jan;153(1):13-9. doi: 10.1093/jb/mvs136. Epub 2012 Nov 21. J Biochem. 2013. PMID: 23172303 Free PMC article. Review.
-
Comparative structure-function analysis of VEGFR-1 and VEGFR-2: What have we learned from chimeric systems?Ann N Y Acad Sci. 2003 May;995:200-7. doi: 10.1111/j.1749-6632.2003.tb03223.x. Ann N Y Acad Sci. 2003. PMID: 12814952 Review.
Cited by
-
Association between genetic variations of vascular endothelial growth factor receptor 2 and glioma in the Chinese Han population.J Mol Neurosci. 2012 Jul;47(3):448-57. doi: 10.1007/s12031-012-9705-9. Epub 2012 Jan 25. J Mol Neurosci. 2012. PMID: 22274884
-
KLK3/PSA and cathepsin D activate VEGF-C and VEGF-D.Elife. 2019 May 17;8:e44478. doi: 10.7554/eLife.44478. Elife. 2019. PMID: 31099754 Free PMC article.
-
Molecular features of interaction between VEGFA and anti-angiogenic drugs used in retinal diseases: a computational approach.Front Pharmacol. 2015 Oct 29;6:248. doi: 10.3389/fphar.2015.00248. eCollection 2015. Front Pharmacol. 2015. PMID: 26578958 Free PMC article.
-
Biophysical Studies of the Induced Dimerization of Human VEGF Receptor 1 Binding Domain by Divalent Metals Competing with VEGF-A.PLoS One. 2016 Dec 12;11(12):e0167755. doi: 10.1371/journal.pone.0167755. eCollection 2016. PLoS One. 2016. PMID: 27942001 Free PMC article.
-
Preliminary data of VEGF-A and VEGFR-2 polymorphisms as predictive factors of radiological response and clinical outcome in iodine-refractory differentiated thyroid cancer treated with sorafenib.Endocrine. 2017 Sep;57(3):539-543. doi: 10.1007/s12020-016-1200-6. Epub 2016 Dec 16. Endocrine. 2017. PMID: 27981515 No abstract available.
References
-
- Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–165. - PubMed
-
- Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. - PubMed
-
- Kärkkäinen MJ, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

