Tumor detection by imaging proteolytic activity
- PMID: 20145119
- PMCID: PMC2823079
- DOI: 10.1158/0008-5472.CAN-09-1640
Tumor detection by imaging proteolytic activity
Abstract
The cell surface protease membrane-type serine protease-1 (MT-SP1), also known as matriptase, is often upregulated in epithelial cancers. We hypothesized that dysregulation of MT-SP1 with regard to its cognate inhibitor hepatocyte growth factor activator inhibitor-1 (HAI-1), a situation that increases proteolytic activity, might be exploited for imaging purposes to differentiate malignant from normal tissue. In this study, we show that MT-SP1 is active on cancer cells and that its activity may be targeted in vivo for tumor detection. A proteolytic activity assay with several MT-SP1-positive human cancer cell lines showed that MT-SP1 antibodies that inhibit recombinant enzyme activity in vitro also bind and inhibit the full-length enzyme expressed on cells. In contrast, in the same assay, MT-SP1-negative cancer cell lines were inactive. Fluorescence microscopy confirmed the cell surface localization of labeled antibodies bound to MT-SP1-positive cells. To evaluate in vivo targeting capability, 0.7 to 2 nmoles of fluorescently labeled antibodies were administered to mice bearing tumors that were positive or negative for MT-SP1. Antibodies localized to MT-SP1-positive tumors (n = 3), permitting visualization of MT-SP1 activity, whereas MT-SP1-negative tumors (n = 2) were not visualized. Our findings define MT-SP1 activity as a useful biomarker to visualize epithelial cancers using a noninvasive antibody-based method.
Conflict of interest statement
The authors declare no competing commercial interest with the work presented here.
Figures

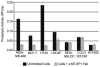
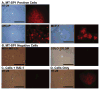
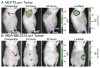
References
-
- Kelloff GJ, Krohn KA, Larson SM, Weissleder R, Mankoff DA, Hoffman JM, et al. The progress and promise of molecular imaging probes in oncologic drug development. Clin Cancer Res. 2005;11(22):7967–85. - PubMed
-
- Mignatti P, Rifkin DB. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993;73(1):161–95. - PubMed
-
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–74. - PubMed
-
- Jedeszko C, Sloane BF. Cysteine cathepsins in human cancer. Biol Chem. 2004;385(11):1017–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

