Regulation of p53 family member isoform DeltaNp63alpha by the nuclear factor-kappaB targeting kinase IkappaB kinase beta
- PMID: 20145131
- PMCID: PMC2963198
- DOI: 10.1158/0008-5472.CAN-09-2613
Regulation of p53 family member isoform DeltaNp63alpha by the nuclear factor-kappaB targeting kinase IkappaB kinase beta
Abstract
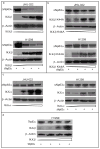
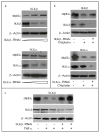
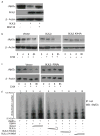
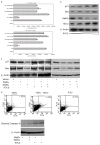
The p53 family gene p63 plays an instrumental role in cellular stress responses including responses to DNA damage. In addition to encoding a full-length transcriptional activator, p63 also encodes several dominant inhibitory isoforms including the isoform DeltaNp63alpha, the function of which is not fully understood. DeltaNp63alpha is degraded in response to DNA damage, thereby enabling an effective cellular response to genotoxic agents. Here, we identify a key molecular mechanism underlying regulation of DeltaNp63alpha expression in response to chemotherapeutic agents or tumor necrosis factor-alpha. We found that DeltaNp63alpha interacts with IkappaB kinase (IKK), a multisubunit protein kinase that consists of two catalytic subunits, IKKalpha and IKKbeta, and a regulatory subunit, IKKgamma. The IKKbeta kinase promotes ubiquitin-mediated proteasomal degradation of DeltaNp63alpha, whereas a kinase-deficient mutant IKKbeta-K44A fails to do so. Cytokine- or chemotherapy-induced stimulation of IKKbeta caused degradation of DeltaNp63alpha and augmented transactivation of p53 family-induced genes involved in the cellular response to DNA damage. Conversely, IKKbeta inhibition attenuated cytokine- or chemotherapy-induced degradation of DeltaNp63alpha. Our findings show that IKKbeta plays an essential role in regulating DeltaNp63alpha in response to extrinsic stimuli. IKK activation represents one mechanism by which levels of DeltaNp63alpha can be reduced, thereby rendering cells susceptible to cell death in the face of cellular stress or DNA damage.
Figures

Similar articles
-
Regulation of ΔNp63α by NFκΒ.Cell Cycle. 2010 Dec 15;9(24):4841-7. doi: 10.4161/cc.9.24.14093. Epub 2010 Dec 15. Cell Cycle. 2010. PMID: 21088498 Free PMC article.
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
U-box-type ubiquitin E4 ligase, UFD2a attenuates cisplatin mediated degradation of DeltaNp63alpha.Cell Cycle. 2008 May 1;7(9):1231-7. doi: 10.4161/cc.7.9.5795. Epub 2008 Feb 19. Cell Cycle. 2008. PMID: 18418053 Free PMC article.
-
The deubiquitinating enzyme USP11 controls an IkappaB kinase alpha (IKKalpha)-p53 signaling pathway in response to tumor necrosis factor alpha (TNFalpha).J Biol Chem. 2007 Nov 23;282(47):33943-8. doi: 10.1074/jbc.M706282200. Epub 2007 Sep 26. J Biol Chem. 2007. PMID: 17897950
-
Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
Cited by
-
A microRNA-dependent circuit controlling p63/p73 homeostasis: p53 family cross-talk meets therapeutic opportunity.Oncotarget. 2011 Mar;2(3):259-64. doi: 10.18632/oncotarget.244. Oncotarget. 2011. PMID: 21436470 Free PMC article.
-
DeltaNp63alpha confers tumor cell resistance to cisplatin through the AKT1 transcriptional regulation.Cancer Res. 2011 Feb 1;71(3):1167-76. doi: 10.1158/0008-5472.CAN-10-1481. Epub 2011 Jan 25. Cancer Res. 2011. PMID: 21266360 Free PMC article.
-
OGDHL is a modifier of AKT-dependent signaling and NF-κB function.PLoS One. 2012;7(11):e48770. doi: 10.1371/journal.pone.0048770. Epub 2012 Nov 12. PLoS One. 2012. PMID: 23152800 Free PMC article.
-
ΔNp63 versatilely regulates a Broad NF-κB gene program and promotes squamous epithelial proliferation, migration, and inflammation.Cancer Res. 2011 May 15;71(10):3688-700. doi: 10.1158/0008-5472.CAN-10-3445. Cancer Res. 2011. PMID: 21576089 Free PMC article.
-
Role of p63 in Development, Tumorigenesis and Cancer Progression.Cancer Microenviron. 2012 Dec;5(3):311-22. doi: 10.1007/s12307-012-0116-9. Epub 2012 Jul 31. Cancer Microenviron. 2012. PMID: 22847008 Free PMC article.
References
-
- Muller M, Schleithoff ES, Stremmel W, et al. One, two, three--p53, p63, p73 and chemosensitivity. Drug Resist Updat. 2006;9:288–306. - PubMed
-
- Strano S, Dell’Orso S, Mongiovi AM, et al. Mutant p53 proteins: between loss and gain of function. Head Neck. 2007;29:488–96. - PubMed
-
- Kaghad M, Bonnet H, Yang A, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–19. - PubMed
-
- Yang A, Kaghad M, Wang Y, et al. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

