Heterogeneous nuclear ribonucleoprotein H blocks MST2-mediated apoptosis in cancer cells by regulating A-Raf transcription
- PMID: 20145135
- PMCID: PMC2880479
- DOI: 10.1158/0008-5472.CAN-09-2740
Heterogeneous nuclear ribonucleoprotein H blocks MST2-mediated apoptosis in cancer cells by regulating A-Raf transcription
Abstract
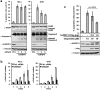
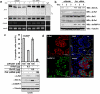
A-Raf belongs to the family of oncogenic Raf kinases that are involved in mitogenic signaling by activating the mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase (MEK)-ERK pathway. Low kinase activity of A-Raf toward MEK suggested that A-Raf might have alternative functions. Here, we show that A-Raf prevents cancer cell apoptosis contingent on the expression of the heterogeneous nuclear ribonucleoprotein H (hnRNP H) splice factor, which is required for the correct transcription and expression of a-raf. Apoptosis was prevented by A-Raf through sequestration and inactivation of the proapoptotic MST2 kinase. Small interfering RNA-mediated knockdown of hnRNP H or A-Raf resulted in MST2-dependent apoptosis. In contrast, enforced expression of either hnRNP H or A-Raf partially counteracted apoptosis induced by etoposide. In vivo expression studies of colon specimens corroborated the overexpression of hnRNP H in malignant tissues and its correlation with A-Raf levels. Our findings define a novel mechanism that is usurped in tumor cells to escape naturally imposed apoptotic signals.
Figures

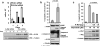


Similar articles
-
Differential localization of A-Raf regulates MST2-mediated apoptosis during epithelial differentiation.Cell Death Differ. 2016 Aug;23(8):1283-95. doi: 10.1038/cdd.2016.2. Epub 2016 Feb 19. Cell Death Differ. 2016. PMID: 26891695 Free PMC article.
-
c-Myc regulates RNA splicing of the A-Raf kinase and its activation of the ERK pathway.Cancer Res. 2011 Jul 1;71(13):4664-74. doi: 10.1158/0008-5472.CAN-10-4447. Epub 2011 Apr 21. Cancer Res. 2011. PMID: 21512137 Free PMC article.
-
A-Raf: A new star of the family of raf kinases.Crit Rev Biochem Mol Biol. 2015;50(6):520-31. doi: 10.3109/10409238.2015.1102858. Epub 2015 Oct 27. Crit Rev Biochem Mol Biol. 2015. PMID: 26508523 Review.
-
Proapoptotic kinase MST2 coordinates signaling crosstalk between RASSF1A, Raf-1, and Akt.Cancer Res. 2010 Feb 1;70(3):1195-203. doi: 10.1158/0008-5472.CAN-09-3147. Epub 2010 Jan 19. Cancer Res. 2010. PMID: 20086174 Free PMC article.
-
Taming the Hippo: Raf-1 controls apoptosis by suppressing MST2/Hippo.Cell Cycle. 2005 Mar;4(3):365-7. doi: 10.4161/cc.4.3.1531. Epub 2005 Mar 8. Cell Cycle. 2005. PMID: 15701972 Review.
Cited by
-
Construction and validation of a novel and superior protein risk model for prognosis prediction in esophageal cancer.Front Genet. 2022 Nov 15;13:1055202. doi: 10.3389/fgene.2022.1055202. eCollection 2022. Front Genet. 2022. PMID: 36457747 Free PMC article.
-
The clinical development of MEK inhibitors.Nat Rev Clin Oncol. 2014 Jul;11(7):385-400. doi: 10.1038/nrclinonc.2014.83. Epub 2014 May 20. Nat Rev Clin Oncol. 2014. PMID: 24840079 Review.
-
A membrane-type-1 matrix metalloproteinase (MT1-MMP)-discoidin domain receptor 1 axis regulates collagen-induced apoptosis in breast cancer cells.PLoS One. 2015 Mar 16;10(3):e0116006. doi: 10.1371/journal.pone.0116006. eCollection 2015. PLoS One. 2015. PMID: 25774665 Free PMC article.
-
Spatial regulation of ARAF controls the MST2-Hippo pathway.Small GTPases. 2019 Jul;10(4):243-248. doi: 10.1080/21541248.2017.1288686. Epub 2017 Mar 10. Small GTPases. 2019. PMID: 28281933 Free PMC article.
-
Dysregulation of miR-212 Promotes Castration Resistance through hnRNPH1-Mediated Regulation of AR and AR-V7: Implications for Racial Disparity of Prostate Cancer.Clin Cancer Res. 2016 Apr 1;22(7):1744-56. doi: 10.1158/1078-0432.CCR-15-1606. Epub 2015 Nov 9. Clin Cancer Res. 2016. PMID: 26553749 Free PMC article.
References
-
- Honore B, Rasmussen HH, Vorum H, et al. Heterogeneous nuclear ribonucleoproteins H, H′, and F are members of a ubiquitously expressed subfamily of related but distinct proteins encoded by genes mapping to different chromosomes. J Biol Chem. 1995;270:28780–9. - PubMed
-
- Garneau D, Revil T, Fisette JF, Chabot B. Heterogeneous nuclear ribonucleoprotein F/H proteins modulate the alternative splicing of the apoptotic mediator Bcl-x. J Biol Chem. 2005;280:22641–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

