Phosphorylation of yeast phosphatidylserine synthase by protein kinase A: identification of Ser46 and Ser47 as major sites of phosphorylation
- PMID: 20145252
- PMCID: PMC2857031
- DOI: 10.1074/jbc.M110.100727
Phosphorylation of yeast phosphatidylserine synthase by protein kinase A: identification of Ser46 and Ser47 as major sites of phosphorylation
Abstract
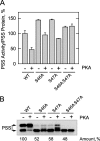
The CHO1-encoded phosphatidylserine synthase from Saccharomyces cerevisiae is phosphorylated and inhibited by protein kinase A in vitro. CHO1 alleles bearing Ser(46) --> Ala and/or Ser(47) --> Ala mutations were constructed and expressed in a cho1Delta mutant lacking phosphatidylserine synthase. In vitro, the S46A/S47A mutation reduced the total amount of phosphorylation by 90% and abolished the inhibitory effect protein kinase A had on phosphatidylserine synthase activity. The enzyme phosphorylation by protein kinase A, which was time- and dose-dependent and dependent on the concentration of ATP, caused a electrophoretic mobility shift from a 27-kDa form to a 30-kDa form. The two electrophoretic forms of phosphatidylserine synthase were present in exponential phase cells, whereas only the 27-kDa form was present in stationary phase cells. In vivo labeling with (32)P(i) and immune complex analysis showed that the 30-kDa form was predominantly phosphorylated when compared with the 27-kDa form. However, the S46A/S47A mutations abolished the protein kinase A-mediated electrophoretic mobility shift. The S46A/S47A mutations also caused a 55% reduction in the total amount of phosphatidylserine synthase in exponential phase cells and a 66% reduction in the amount of enzyme in stationary phase cells. In phospholipid composition analysis, cells expressing the S46A/S47A mutant enzyme exhibited a 57% decrease in phosphatidylserine and a 40% increase in phosphatidylinositol. These results indicate that phosphatidylserine synthase is phosphorylated on Ser(46) and Ser(47) by protein kinase A, which results in a higher amount of enzyme for the net effect of stimulating the synthesis of phosphatidylserine.
Figures



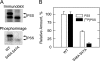

Similar articles
-
Phosphatidate phosphatase regulates membrane phospholipid synthesis via phosphatidylserine synthase.Adv Biol Regul. 2018 Jan;67:49-58. doi: 10.1016/j.jbior.2017.08.001. Epub 2017 Aug 16. Adv Biol Regul. 2018. PMID: 28827025 Free PMC article. Review.
-
Yeast PAH1-encoded phosphatidate phosphatase controls the expression of CHO1-encoded phosphatidylserine synthase for membrane phospholipid synthesis.J Biol Chem. 2017 Aug 11;292(32):13230-13242. doi: 10.1074/jbc.M117.801720. Epub 2017 Jul 3. J Biol Chem. 2017. PMID: 28673963 Free PMC article.
-
Phosphorylation of yeast phosphatidylserine synthase in vivo and in vitro by cyclic AMP-dependent protein kinase.Proc Natl Acad Sci U S A. 1988 Nov;85(21):7962-6. doi: 10.1073/pnas.85.21.7962. Proc Natl Acad Sci U S A. 1988. PMID: 2847149 Free PMC article.
-
Respiratory deficiency mediates the regulation of CHO1-encoded phosphatidylserine synthase by mRNA stability in Saccharomyces cerevisiae.J Biol Chem. 2007 Oct 26;282(43):31217-27. doi: 10.1074/jbc.M705098200. Epub 2007 Aug 30. J Biol Chem. 2007. PMID: 17761681 Free PMC article.
-
Phosphatidylserine synthase from yeast.Biochim Biophys Acta. 1997 Sep 4;1348(1-2):228-35. doi: 10.1016/s0005-2760(97)00102-1. Biochim Biophys Acta. 1997. PMID: 9370337 Review.
Cited by
-
Mapping the Substrate-Binding Sites in the Phosphatidylserine Synthase in Candida albicans.Front Cell Infect Microbiol. 2021 Dec 22;11:765266. doi: 10.3389/fcimb.2021.765266. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 35004345 Free PMC article.
-
PKA regulatory subunit Bcy1 couples growth, lipid metabolism, and fermentation during anaerobic xylose growth in Saccharomyces cerevisiae.PLoS Genet. 2023 Jul 6;19(7):e1010593. doi: 10.1371/journal.pgen.1010593. eCollection 2023 Jul. PLoS Genet. 2023. PMID: 37410771 Free PMC article.
-
Phosphorylation regulates myo-inositol-3-phosphate synthase: a novel regulatory mechanism of inositol biosynthesis.J Biol Chem. 2013 Sep 13;288(37):26822-33. doi: 10.1074/jbc.M113.479121. Epub 2013 Jul 30. J Biol Chem. 2013. PMID: 23902760 Free PMC article.
-
Phosphatidate phosphatase regulates membrane phospholipid synthesis via phosphatidylserine synthase.Adv Biol Regul. 2018 Jan;67:49-58. doi: 10.1016/j.jbior.2017.08.001. Epub 2017 Aug 16. Adv Biol Regul. 2018. PMID: 28827025 Free PMC article. Review.
-
The P4-ATPase Drs2 interacts with and stabilizes the multisubunit tethering complex TRAPPIII in yeast.EMBO Rep. 2023 May 4;24(5):e56134. doi: 10.15252/embr.202256134. Epub 2023 Mar 16. EMBO Rep. 2023. PMID: 36929574 Free PMC article.
References
-
- Bae-Lee M. S., Carman G. M. (1984) J. Biol. Chem. 259, 10857–10862 - PubMed
-
- Nikawa J., Tsukagoshi Y., Kodaki T., Yamashita S. (1987) Eur. J. Biochem. 167, 7–12 - PubMed
-
- Kiyono K., Miura K., Kushima Y., Hikiji T., Fukushima M., Shibuya I., Ohta A. (1987) J. Biochem. 102, 1089–1100 - PubMed
-
- Natter K., Leitner P., Faschinger A., Wolinski H., McCraith S., Fields S., Kohlwein S. D. (2005) Mol. Cell. Proteomics 4, 662–672 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

