Mechanistic differences between two conserved classes of small heat shock proteins found in the plant cytosol
- PMID: 20145254
- PMCID: PMC2857027
- DOI: 10.1074/jbc.M109.074088
Mechanistic differences between two conserved classes of small heat shock proteins found in the plant cytosol
Abstract
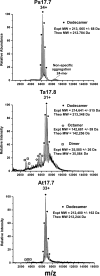
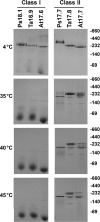
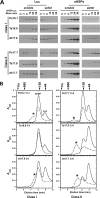
The small heat shock proteins (sHSPs) and alpha-crystallins are highly effective, ATP-independent chaperones that can bind denaturing client proteins to prevent their irreversible aggregation. One model of sHSP function suggests that the oligomeric sHSPs are activated to the client-binding form by dissociation at elevated temperatures to dimers or other sub-oligomeric species. Here we examine this model in a comparison of the oligomeric structure and chaperone activity of two conserved classes of cytosolic sHSPs in plants, the class I (CI) and class II (CII) proteins. Like the CI sHSPs, recombinant CII sHSPs from three divergent plant species, pea, wheat, and Arabidopsis, are dodecamers as determined by nano-electrospray mass spectrometry. While at 35 to 45 degrees C, all three CI sHSPs reversibly dissociate to dimers, the CII sHSPs retain oligomeric structure at high temperature. The CII dodecamers are, however, dynamic and rapidly exchange subunits, but unlike CI sHSPs, the exchange unit appears larger than a dimer. Differences in dodecameric structure are also reflected in the fact that the CII proteins do not hetero-oligomerize with CI sHSPs. Binding of the hydrophobic probe bis-ANS and limited proteolysis demonstrate CII proteins undergo significant, reversible structural changes at high temperature. All three recombinant CII proteins more efficiently protect firefly luciferase from insolubilization during heating than do the CI proteins. The CI and CII proteins behave strictly additively in client protection. In total, the results demonstrate that different sHSPs can achieve effective protection of client proteins by varied mechanisms.
Figures
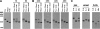



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

