Atenolol pharmacokinetics and excretion in breast milk during the first 6 to 8 months postpartum
- PMID: 20145263
- PMCID: PMC2940977
- DOI: 10.1177/0091270009358708
Atenolol pharmacokinetics and excretion in breast milk during the first 6 to 8 months postpartum
Abstract
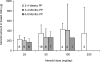
The objectives were to evaluate the time course for atenolol pharmacokinetics in lactating women postpartum and to quantify atenolol plasma concentrations in the women's 3- to 4-month-old nursing infants. Data were collected during 1 dosing interval from lactating women treated with atenolol for therapeutic reasons, at 2 to 4 weeks (n = 32), 3 to 4 months (n = 22), and 6 to 8 months (n = 17) postpartum. A single blood sample was collected from 15 nursing infants (3-4 months of age) of the mothers participating in the study. At 2 to 4 weeks, 3 to 4 months, and 6 to 8 months postpartum, atenolol infant doses, relative to the mother's weight-adjusted dose, were 14.6% ± 7.6%, 8.3% ± 5.2% and 5.9% ± 2.9%, respectively. Over this time, maternal atenolol pharmacokinetics did not change to a clinically significant extent. Atenolol concentrations were below assay quantification limits (<10 ng/mL) in the plasma of all 3- to 4-month-old nursing infants studied. These findings support the careful use of atenolol during breastfeeding, because in the vast majority of healthy, term infants, atenolol concentrations will be too low to be clinically relevant. Premature infants and those with kidney disease require further study. Infant exposure depends on maternal dose and decreases during the first 6 to 8 months postpartum.
Figures
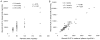
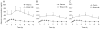
References
-
- American Academy of Pediatrics, Committee on Drugs. The transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776–779. - PubMed
-
- TenorminR product labeling. AstraZeneca; Wilmington DE: Apr 08, 2008.
-
- Schmimmel MS, Eidelman AJ, Wilschanski MA, Shaw D, Ogilvie RJ, Koren G. Toxic effects of atenolol consumed during breast feeding. J Pediatr. 1989;114:476–478. - PubMed
-
- Fowler MB, Brudenell M, Jackson G, Holt DW. Essential hypertension and pregnancy: successful outcome with atenolol. Br J Clin Pract. 1984;38:73–74. - PubMed
-
- White WB, Andreoli JW, Wong SH, Cohn RD. Atenolol in human plasma and breast milk. Obstet Gynecol. 1984;63(3 Suppl):42S–44S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

