Hematopoietic stem cells in co-culture with mesenchymal stromal cells--modeling the niche compartments in vitro
- PMID: 20145267
- PMCID: PMC2857183
- DOI: 10.3324/haematol.2009.010736
Hematopoietic stem cells in co-culture with mesenchymal stromal cells--modeling the niche compartments in vitro
Abstract
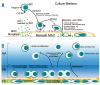
Background: Hematopoietic stem cells located in the bone marrow interact with a specific microenvironment referred to as the stem cell niche. Data derived from ex vivo co-culture systems using mesenchymal stromal cells as a feeder cell layer suggest that cell-to-cell contact has a significant impact on the expansion, migratory potential and 'stemness' of hematopoietic stem cells. Here we investigated in detail the spatial relationship between hematopoietic stem cells and mesenchymal stromal cells during ex vivo expansion.
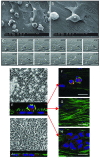
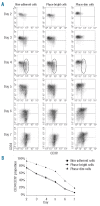
Design and methods: In the co-culture system, we defined three distinct localizations of hematopoietic stem cells relative to the mesenchymal stromal cell layer: (i) those in supernatant (non-adherent cells); (ii) those adhering to the surface of mesenchymal stromal cells (phase-bright cells) and (iii) those beneath the mesenchymal stromal cells (phase-dim cells). Cell cycle, proliferation, cell division and immunophenotype of these three cell fractions were evaluated from day 1 to 7.
Results: Phase-bright cells contained the highest proportion of cycling progenitors during co-culture. In contrast, phase-dim cells divided much more slowly and retained a more immature phenotype compared to the other cell fractions. The phase-dim compartment was soon enriched for CD34(+)/CD38(-) cells. Migration beneath the mesenchymal stromal cell layer could be hampered by inhibiting integrin beta1 or CXCR4.
Conclusions: Our data suggest that the mesenchymal stromal cell surface is the predominant site of proliferation of hematopoietic stem cells, whereas the compartment beneath the mesenchymal stromal cell layer seems to mimic the stem cell niche for more immature cells. The SDF-1/CXCR4 interaction and integrin-mediated cell adhesion play important roles in the distribution of hematopoietic stem cells in the co-culture system.
Figures



References
-
- Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273(5272):242–5. - PubMed
-
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404(6774):193–7. - PubMed
-
- Metcalf D. On hematopoietic stem cell fate. Immunity. 2007;26(6):669–73. - PubMed
-
- Gordon MY. Stem cells for regenerative medicine – biological attributes and clinical application. Exp Hematol. 2008;36(6):726–32. - PubMed
-
- Sorrentino BP. Clinical strategies for expansion of haematopoietic stem cells. Nat Rev Immunol. 2004;4(11):878–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

