A new MAP kinase protein involved in estradiol-stimulated reproduction of the helminth parasite Taenia crassiceps
- PMID: 20145710
- PMCID: PMC2817376
- DOI: 10.1155/2010/747121
A new MAP kinase protein involved in estradiol-stimulated reproduction of the helminth parasite Taenia crassiceps
Abstract
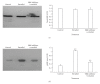
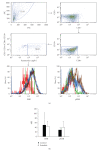
MAP kinases (MAPK) are involved in the regulation of cellular processes such as reproduction and growth. In parasites, the role of MAPK has been scarcely studied. Here, we describe the participation of an ERK-like protein in estrogen-dependent reproduction of the helminth parasite Taenia crassiceps. Our results show that 17beta-estradiol induces a concentration-dependent increase in the bud number of in vitro cultured cysticerci. If parasites are also incubated in presence of an ERK-inhibitor, the stimulatory effect of estrogen is blocked. The expression of ERK-like mRNA and its corresponding protein was detected in the parasite. The ERK-like protein was over-expressed by all treatments. Nevertheless, a strong induction of phosphorylation of this protein was observed only in response to 17beta-estradiol. Cross-contamination by host cells was discarded by flow cytometry analysis. Parasite cells expressing the ERK-like protein were exclusively located at the subtegument tissue by confocal microscopy. Finally, the ERK-like protein was separated by bidimensional electrophoresis and then sequenced, showing the conserved TEY activation motif, typical of all known ERK 1/2 proteins. Our results show that an ERK-like protein is involved in the molecular signalling during the interaction between the host and T. crassiceps, and may be considered as target for anti-helminth drugs design.
Figures




Similar articles
-
A helminth cestode parasite express an estrogen-binding protein resembling a classic nuclear estrogen receptor.Steroids. 2011 Sep-Oct;76(10-11):1149-59. doi: 10.1016/j.steroids.2011.05.003. Epub 2011 May 19. Steroids. 2011. PMID: 21621550
-
Steroid synthesis by Taenia crassiceps WFU cysticerci is regulated by enzyme inhibitors.Gen Comp Endocrinol. 2013 Jul 1;188:212-7. doi: 10.1016/j.ygcen.2013.03.034. Epub 2013 Apr 20. Gen Comp Endocrinol. 2013. PMID: 23608546
-
A novel progesterone receptor membrane component (PGRMC) in the human and swine parasite Taenia solium: implications to the host-parasite relationship.Parasit Vectors. 2018 Mar 9;11(1):161. doi: 10.1186/s13071-018-2703-1. Parasit Vectors. 2018. PMID: 29523160 Free PMC article.
-
Steroid hormone production by parasites: the case of Taenia crassiceps and Taenia solium cysticerci.J Steroid Biochem Mol Biol. 2003 Jun;85(2-5):221-5. doi: 10.1016/s0960-0760(03)00233-4. J Steroid Biochem Mol Biol. 2003. PMID: 12943707 Review.
-
Parasite regulation by host hormones: an old mechanism of host exploitation?Trends Parasitol. 2005 Dec;21(12):588-93. doi: 10.1016/j.pt.2005.09.013. Epub 2005 Oct 19. Trends Parasitol. 2005. PMID: 16236553 Review.
Cited by
-
In vitro system for the growth and asexual multiplication of Taenia crassiceps cysticerci.Parasitology. 2022 Nov;149(13):1775-1780. doi: 10.1017/S0031182022001354. Epub 2022 Sep 27. Parasitology. 2022. PMID: 36165285 Free PMC article.
-
Protein kinase C and extracellular signal-regulated kinase regulate movement, attachment, pairing and egg release in Schistosoma mansoni.PLoS Negl Trop Dis. 2014 Jun 12;8(6):e2924. doi: 10.1371/journal.pntd.0002924. eCollection 2014 Jun. PLoS Negl Trop Dis. 2014. PMID: 24921927 Free PMC article.
-
Neurocysticercosis: a review on status in India, management, and current therapeutic interventions.Parasitol Res. 2017 Jan;116(1):21-33. doi: 10.1007/s00436-016-5278-9. Epub 2016 Oct 24. Parasitol Res. 2017. PMID: 27774576 Review.
References
-
- Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends in Biochemical Sciences. 2006;31(5):268–275. - PubMed
-
- Wray S, Noble K. Sex hormones and excitation-contraction coupling in the uterus: the effects of oestrous and hormones. Journal of Neuroendocrinology. 2008;20(4):451–461. - PubMed
-
- Maioli M, Ventura C. Protein kinase C control of gene expression. Critical Reviews in Eukaryotic Gene Expression. 2001;11(1–3):243–267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

