Linking ER Stress to Autophagy: Potential Implications for Cancer Therapy
- PMID: 20145727
- PMCID: PMC2817393
- DOI: 10.1155/2010/930509
Linking ER Stress to Autophagy: Potential Implications for Cancer Therapy
Abstract
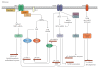
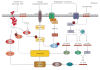
Different physiological and pathological conditions can perturb protein folding in the endoplasmic reticulum, leading to a condition known as ER stress. ER stress activates a complex intracellular signal transduction pathway, called unfolded protein response (UPR). The UPR is tailored essentially to reestablish ER homeostasis also through adaptive mechanisms involving the stimulation of autophagy. However, when persistent, ER stress can switch the cytoprotective functions of UPR and autophagy into cell death promoting mechanisms. Recently, a variety of anticancer therapies have been linked to the induction of ER stress in cancer cells, suggesting that strategies devised to stimulate its prodeath function or block its prosurvival function, could be envisaged to improve their tumoricidial action. A better understanding of the molecular mechanisms that determine the final outcome of UPR and autophagy activation by chemotherapeutic agents, will offer new opportunities to improve existing cancer therapies as well as unravel novel targets for cancer treatment.
Figures

Similar articles
-
Detecting autophagy in response to ER stress signals in cancer.Methods Enzymol. 2011;489:297-317. doi: 10.1016/B978-0-12-385116-1.00017-0. Methods Enzymol. 2011. PMID: 21266237
-
Oncogenic BRAF, endoplasmic reticulum stress, and autophagy: Crosstalk and therapeutic targets in cutaneous melanoma.Mutat Res Rev Mutat Res. 2020 Jul-Sep;785:108321. doi: 10.1016/j.mrrev.2020.108321. Epub 2020 Jul 7. Mutat Res Rev Mutat Res. 2020. PMID: 32800272 Review.
-
Crosstalk between endoplasmic reticulum stress response and autophagy in human diseases.Anim Cells Syst (Seoul). 2023 Feb 23;27(1):29-37. doi: 10.1080/19768354.2023.2181217. eCollection 2023. Anim Cells Syst (Seoul). 2023. PMID: 36860271 Free PMC article. Review.
-
Cell intrinsic and extrinsic activators of the unfolded protein response in cancer: Mechanisms and targets for therapy.Semin Cancer Biol. 2015 Aug;33:3-15. doi: 10.1016/j.semcancer.2015.04.002. Epub 2015 Apr 25. Semin Cancer Biol. 2015. PMID: 25920797 Free PMC article. Review.
-
Interplay between Endoplasmic Reticulum (ER) Stress and Autophagy Induces Mutant p53H273 Degradation.Biomolecules. 2020 Mar 3;10(3):392. doi: 10.3390/biom10030392. Biomolecules. 2020. PMID: 32138264 Free PMC article.
Cited by
-
JNK and macroautophagy activation by bortezomib has a pro-survival effect in primary effusion lymphoma cells.PLoS One. 2013 Sep 26;8(9):e75965. doi: 10.1371/journal.pone.0075965. eCollection 2013. PLoS One. 2013. PMID: 24086672 Free PMC article.
-
Dengue virus-induced ER stress is required for autophagy activation, viral replication, and pathogenesis both in vitro and in vivo.Sci Rep. 2018 Jan 11;8(1):489. doi: 10.1038/s41598-017-18909-3. Sci Rep. 2018. PMID: 29323257 Free PMC article.
-
Swimming Differentially Affects T2DM-Induced Skeletal Muscle ER Stress and Mitochondrial Dysfunction Related to MAM.Diabetes Metab Syndr Obes. 2020 Apr 30;13:1417-1428. doi: 10.2147/DMSO.S243024. eCollection 2020. Diabetes Metab Syndr Obes. 2020. PMID: 32431525 Free PMC article.
-
Sustained conditional knockdown reveals intracellular bone sialoprotein as essential for breast cancer skeletal metastasis.Oncotarget. 2014 Jul 30;5(14):5510-22. doi: 10.18632/oncotarget.2132. Oncotarget. 2014. PMID: 24980816 Free PMC article.
-
An industrialized diet as a determinant of methylation in the 1F region of the NR3C1 gene promoter.Front Nutr. 2024 Apr 3;11:1168715. doi: 10.3389/fnut.2024.1168715. eCollection 2024. Front Nutr. 2024. PMID: 38633601 Free PMC article.
References
-
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32(5-6):235–249. - PubMed
-
- Görlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxidants and Redox Signaling. 2006;8(9-10):1391–1418. - PubMed
-
- Sevier CS, Kaiser CA. Ero1 and redox homeostasis in the endoplasmic reticulum. Biochimica et Biophysica Acta. 2008;1783(4):549–556. - PubMed
LinkOut - more resources
Full Text Sources

