Mode of antiviral action of silver nanoparticles against HIV-1
- PMID: 20145735
- PMCID: PMC2818642
- DOI: 10.1186/1477-3155-8-1
Mode of antiviral action of silver nanoparticles against HIV-1
Abstract
Background: Silver nanoparticles have proven to exert antiviral activity against HIV-1 at non-cytotoxic concentrations, but the mechanism underlying their HIV-inhibitory activity has not been not fully elucidated. In this study, silver nanoparticles are evaluated to elucidate their mode of antiviral action against HIV-1 using a panel of different in vitro assays.
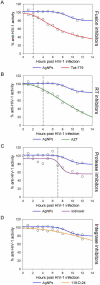
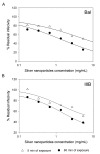
Results: Our data suggest that silver nanoparticles exert anti-HIV activity at an early stage of viral replication, most likely as a virucidal agent or as an inhibitor of viral entry. Silver nanoparticles bind to gp120 in a manner that prevents CD4-dependent virion binding, fusion, and infectivity, acting as an effective virucidal agent against cell-free virus (laboratory strains, clinical isolates, T and M tropic strains, and resistant strains) and cell-associated virus. Besides, silver nanoparticles inhibit post-entry stages of the HIV-1 life cycle.
Conclusions: These properties make them a broad-spectrum agent not prone to inducing resistance that could be used preventively against a wide variety of circulating HIV-1 strains.
Figures


References
-
- Joint United Nations Programme on HIV AIDS (UNAIDS) Report on the global AIDS epidemic. Geneva, Switzerland. 2008.
-
- Vercauteren J, Deforche K, Theys K, Debruyne M, Duque LM, Peres S, Carvalho AP, Mansinho K, Vandamme AM, Camacho R. The incidence of multidrug and full class resistance in HIV-1 infected patients is decreasing over time (2001-2006) in Portugal. Retrovirology. 2008;5:12. doi: 10.1186/1742-4690-5-12. - DOI - PMC - PubMed
-
- Lin PF, Blair W, Wang T, Spicer T, Guo Q, Zhou N, Gong YF, Wang HG, Rose R, Yamanaka G, Robinson B, Li CB, Fridell R, Deminie C, Demers G, Yang Z, Zadjura L, Meanwell N, Colonno R. A small molecule HIV-1 inhibitor that targets the HIV-1 envelope and inhibits CD4 receptor binding. Proc Natl Acad Sci USA. 2003;100:11013–11018. doi: 10.1073/pnas.1832214100. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

