Controlled embryoid body formation via surface modification and avidin-biotin cross-linking
- PMID: 20145998
- PMCID: PMC2825297
- DOI: 10.1007/s10616-010-9255-3
Controlled embryoid body formation via surface modification and avidin-biotin cross-linking
Abstract
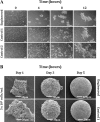
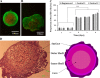
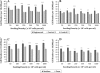
Cell-cell interaction is an integral part of embryoid body (EB) formation controlling 3D aggregation. Manipulation of embryonic stem (ES) cell interactions could provide control over EB formation. Studies have shown a direct relationship between EB formation and ES cell differentiation. We have previously described a cell surface modification and cross-linking method for influencing cell-cell interaction and formation of multicellular constructs. Here we show further characterisation of this engineered aggregation. We demonstrate that engineering accelerates ES cell aggregation, forming larger, denser and more stable EBs than control samples, with no significant decrease in constituent ES cell viability. However, extended culture >/=5 days reveals significant core necrosis creating a layered EB structure. Accelerated aggregation through engineering circumvents this problem as EB formation time is reduced. We conclude that the proposed engineering method influences initial ES cell-ES cell interactions and EB formation. This methodology could be employed to further our understanding of intrinsic EB properties and their effect on ES cell differentiation.
Figures


Similar articles
-
[Scalable production of embryoid bodies with the rotay cell culture system.].Sheng Li Xue Bao. 2005 Aug 25;57(4):486-92. Sheng Li Xue Bao. 2005. PMID: 16094497 Chinese.
-
Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems.Biotechnol Bioeng. 2002 May 20;78(4):442-53. doi: 10.1002/bit.10220. Biotechnol Bioeng. 2002. PMID: 11948451
-
Effect of glucose concentration during embryoid body (EB) formation from mouse embryonic stem cells on EB growth and cell differentiation.J Biosci Bioeng. 2011 Jan;111(1):92-7. doi: 10.1016/j.jbiosc.2010.09.001. Epub 2010 Sep 24. J Biosci Bioeng. 2011. PMID: 20869914
-
Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells.J Biosci Bioeng. 2007 May;103(5):389-98. doi: 10.1263/jbb.103.389. J Biosci Bioeng. 2007. PMID: 17609152 Review.
-
Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation.Biotechnol Prog. 2009 Jan-Feb;25(1):43-51. doi: 10.1002/btpr.139. Biotechnol Prog. 2009. PMID: 19198003 Free PMC article. Review.
Cited by
-
Controlled Self-assembly of Stem Cell Aggregates Instructs Pluripotency and Lineage Bias.Sci Rep. 2017 Oct 25;7(1):14070. doi: 10.1038/s41598-017-14325-9. Sci Rep. 2017. PMID: 29070799 Free PMC article.
-
Cell Mechanics in Embryoid Bodies.Cells. 2020 Oct 11;9(10):2270. doi: 10.3390/cells9102270. Cells. 2020. PMID: 33050550 Free PMC article. Review.
-
Using Advanced Cell Culture Techniques to Differentiate Pluripotent Stem Cells and Recreate Tissue Structures Representative of Teratoma Xenografts.Front Cell Dev Biol. 2021 May 6;9:667246. doi: 10.3389/fcell.2021.667246. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34026759 Free PMC article.
-
Multicellular Liver Organoids: Generation and Importance of Diverse Specialized Cellular Components.Cells. 2023 May 19;12(10):1429. doi: 10.3390/cells12101429. Cells. 2023. PMID: 37408262 Free PMC article. Review.
-
Functional maintenance of differentiated embryoid bodies in microfluidic systems: a platform for personalized medicine.Stem Cells Transl Med. 2015 Mar;4(3):261-8. doi: 10.5966/sctm.2014-0119. Epub 2015 Feb 9. Stem Cells Transl Med. 2015. PMID: 25666845 Free PMC article.
References
-
- Abilez O, Benharash P, Mehrotra M, Miyamoto E, Gale A, Picquet J, Xu C, Zarins C. A novel culture system shows that stem cells can be grown in 3D and under physiologic pulsatile conditions for tissue engineering of vascular grafts. J Surg Res. 2006;132:170–178. doi: 10.1016/j.jss.2006.02.017. - DOI - PubMed
-
- Bielby RC, Boccaccini AR, Polak JM, Buttery LDK. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 2004;10:1518–1528. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

