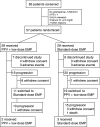
A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer
- PMID: 20146063
- PMCID: PMC11030921
- DOI: 10.1007/s00262-010-0822-4
A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer
Abstract
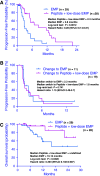
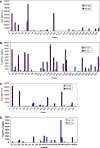
Personalized peptide vaccination (PPV) combined with chemotherapy could be a novel approach for many cancer patients. In this randomized study, we evaluated the anti-tumor effect and safety of PPV plus low-dose estramustine phosphate (EMP) as compared to standard-dose EMP for HLA-A2- or -A24-positive patients with castration resistant prostate cancer. Patients were randomized into groups receiving either PPV plus low-dose EMP (280 mg/day) or standard-dose EMP (560 mg/day). After disease progression, patients were switched to the opposite regime. The primary end point was progression-free survival (PFS). We randomly assigned 28 patients to receive PPV plus low-dose EMP and 29 patients to receive standard-dose EMP. Nineteen events in the PPV group and 20 events in the EMP group occurred during the first treatment. Median PFS for the first treatment was 8.5 months in the PPV group and 2.8 months in the EMP group with a hazard ratio (HR) of 0.28 (95% CI, 0.14-0.61; log-rank P = 0.0012), while there was no difference for median PFS for the second treatment. The HR for overall survival was 0.3 (95% CI, 0.1-0.91) in favor of the PPV plus low-dose EMP group (log-rank, P = 0.0328). The PPV plus low-dose EMP was well tolerated without major adverse effects and with increased levels of IgG and cytotoxic-T cell responses to the vaccinated peptides. PPV plus low-dose EMP was associated with an improvement in PSA-based PFS as compared to the standard-dose EMP alone.
Figures
Comment in
-
Prostate cancer. Immunotherapy and combined chemotherapy for castration-resistant and metastatic disease.Nat Rev Urol. 2010 Sep;7(9):472. doi: 10.1038/nrurol.2010.124. Nat Rev Urol. 2010. PMID: 20839381 No abstract available.
Similar articles
-
Addition of estramustine to chemotherapy and survival of patients with castration-refractory prostate cancer: a meta-analysis of individual patient data.Lancet Oncol. 2007 Nov;8(11):994-1000. doi: 10.1016/S1470-2045(07)70284-X. Epub 2007 Oct 17. Lancet Oncol. 2007. PMID: 17942366
-
A phase I study of personalized peptide vaccination using 14 kinds of vaccine in combination with low-dose estramustine in HLA-A24-positive patients with castration-resistant prostate cancer.Prostate. 2011 Apr;71(5):470-9. doi: 10.1002/pros.21261. Epub 2010 Sep 28. Prostate. 2011. PMID: 20878951 Clinical Trial.
-
Effective and Safe Administration of Low-Dose Estramustine Phosphate for Castration-Resistant Prostate Cancer.Clin Genitourin Cancer. 2016 Feb;14(1):e9-e17. doi: 10.1016/j.clgc.2015.08.008. Epub 2015 Sep 2. Clin Genitourin Cancer. 2016. PMID: 26433627 Clinical Trial.
-
EMP combination chemotherapy and low-dose monotherapy in advanced prostate cancer.Expert Rev Anticancer Ther. 2002 Feb;2(1):59-71. doi: 10.1586/14737140.2.1.59. Expert Rev Anticancer Ther. 2002. PMID: 12113067 Review.
-
The use of estramustine phosphate in the modern management of advanced prostate cancer.BJU Int. 2011 Dec;108(11):1782-6. doi: 10.1111/j.1464-410X.2011.10201.x. Epub 2011 Jul 14. BJU Int. 2011. PMID: 21756277 Review.
Cited by
-
Personalized peptide vaccination for cervical cancer patients who have received prior platinum-based chemotherapy.Cancer Sci. 2015 Sep;106(9):1111-7. doi: 10.1111/cas.12729. Epub 2015 Jul 22. Cancer Sci. 2015. PMID: 26122553 Free PMC article. Clinical Trial.
-
Identification of novel Lck-derived T helper epitope long peptides applicable for HLA-A2(+) cancer patients as cancer vaccine.Cancer Sci. 2015 Nov;106(11):1493-8. doi: 10.1111/cas.12805. Epub 2015 Oct 16. Cancer Sci. 2015. PMID: 26331453 Free PMC article.
-
Potential Mechanisms for Immunotherapy Resistance in Adult Soft-Tissue Sarcoma.Target Oncol. 2025 May;20(3):485-502. doi: 10.1007/s11523-025-01145-5. Epub 2025 Apr 27. Target Oncol. 2025. PMID: 40289241 Free PMC article. Review.
-
Vaccines as treatments for prostate cancer.Nat Rev Urol. 2023 Sep;20(9):544-559. doi: 10.1038/s41585-023-00739-w. Epub 2023 Mar 6. Nat Rev Urol. 2023. PMID: 36879114 Free PMC article. Review.
-
Humoral immune responses to EGFR-derived peptides predict progression-free and overall survival of non-small cell lung cancer patients receiving gefitinib.PLoS One. 2014 Jan 31;9(1):e86667. doi: 10.1371/journal.pone.0086667. eCollection 2014. PLoS One. 2014. PMID: 24497964 Free PMC article.
References
-
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA, TAX 327 Investigators Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1488–1490. doi: 10.1056/NEJMoa040720. - DOI - PubMed
-
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. - DOI - PubMed
-
- Ressing ME, van Driel WJ, Brandt RM, Kenter GG, de Jong JH, Bauknecht T, Fleuren GJ, Hoogerhout P, Offringa R, Sette A, Celis E, Grey H, Trimbos BJ, Kast WM, Melief CJ. Detection of T helper responses, but not of human papillomavirus-specific cytotoxic T lymphocyte responses, after peptide vaccination of patients with cervical carcinoma. J Immunother. 2000;23:255–266. doi: 10.1097/00002371-200003000-00010. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

