Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice
- PMID: 20146261
- PMCID: PMC2903010
- DOI: 10.1002/hep.23510
Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice
Abstract
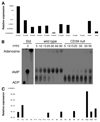
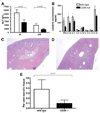
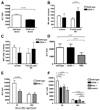
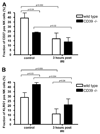
Natural killer (NK) cells play crucial roles in innate immunity and express CD39 (Ecto-nucleoside triphosphate diphosphohydrolase 1 [E-NTPD1]), a rate-limiting ectonucleotidase in the phosphohydrolysis of extracellular nucleotides to adenosine. We have studied the effects of CD39 gene deletion on NK cells in dictating outcomes after partial hepatic ischemia/reperfusion injury (IRI). We show in mice that gene deletion of CD39 is associated with marked decreases in phosphohydrolysis of adenosine triphosphate (ATP) and adenosine diphosphate to adenosine monophosphate on NK cells, thereby modulating the type-2 purinergic (P2) receptors demonstrated on these cells. We note that CD39-null mice are protected from acute vascular injury after single-lobe warm IRI, and, relative to control wild-type mice, display significantly less elevation of aminotransferases with less pronounced histopathological changes associated with IRI. Selective adoptive transfers of immune cells into Rag2/common gamma null mice (deficient in T cells, B cells, and NK/NKT cells) suggest that it is CD39 deletion on NK cells that provides end-organ protection, which is comparable to that seen in the absence of interferon gamma. Indeed, NK effector mechanisms such as interferon gamma secretion are inhibited by P2 receptor activation in vitro. Specifically, ATPgammaS (a nonhydrolyzable ATP analog) inhibits secretion of interferon gamma by NK cells in response to interleukin-12 and interleukin-18, providing a mechanistic link between CD39 deletion and altered cytokine secretion.
Conclusion: We propose that CD39 deficiency and changes in P2 receptor activation abrogate secretion of interferon gamma by NK cells in response to inflammatory mediators, thereby limiting tissue damage mediated by these innate immune cells during IRI.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures

References
-
- Feng L, Cheng F, Ye Z, Li S, He Y, Yao X, et al. The effect of renal ischemia-reperfusion injury on expression of RAE-1 and H60 in mice kidney. Transplant Proc. 2006;38:2195–2198. - PubMed
-
- Kato A, Graul-Layman A, Edwards MJ, Lentsch AB. Promotion of hepatic ischemia/reperfusion injury by IL-12 is independent of STAT4. Transplantation. 2002;73:1142–1145. - PubMed
-
- Takeuchi D, Yoshidome H, Kato A, Ito H, Kimura F, Shimizu H, et al. Interleukin 18 causes hepatic ischemia/reperfusion injury by suppressing anti-inflammatory cytokine expression in mice. HEPATOLOGY. 2004;39:699–710. - PubMed
-
- Friedman DJ, Rennke HG, Csizmadia E, Enjyoji K, Robson SC. The vascular ectonucleotidase ENTPD1 is a novel renoprotective factor in diabetic nephropathy. Diabetes. 2007;56:2371–2379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL057307/HL/NHLBI NIH HHS/United States
- PR043034/PR/OCPHP CDC HHS/United States
- HL57307/HL/NHLBI NIH HHS/United States
- R01 GM060475/GM/NIGMS NIH HHS/United States
- HL076540/HL/NHLBI NIH HHS/United States
- R01 GM051477/GM/NIGMS NIH HHS/United States
- R29 GM051477/GM/NIGMS NIH HHS/United States
- GM-60475/GM/NIGMS NIH HHS/United States
- R01 HL063972/HL/NHLBI NIH HHS/United States
- GM-51477/GM/NIGMS NIH HHS/United States
- U01 AI066331/AI/NIAID NIH HHS/United States
- HL63972/HL/NHLBI NIH HHS/United States
- P01 HL076540/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
