Discovery of 4-(4-(2-((5-Hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)ethyl)piperazin-1-yl)quinolin-8-ol and its analogues as highly potent dopamine D2/D3 agonists and as iron chelator: in vivo activity indicates potential application in symptomatic and neuroprotective therapy for Parkinson's disease
- PMID: 20146482
- PMCID: PMC2844127
- DOI: 10.1021/jm901618d
Discovery of 4-(4-(2-((5-Hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)ethyl)piperazin-1-yl)quinolin-8-ol and its analogues as highly potent dopamine D2/D3 agonists and as iron chelator: in vivo activity indicates potential application in symptomatic and neuroprotective therapy for Parkinson's disease
Abstract
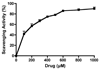
The role of iron in the pathogenesis of Parkinson's disease (PD) has been implicated strongly because of generation of oxidative stress leading to dopamine cell death. In our overall goal to develop bifunctional/multifunctional drugs, we designed dopamine D2/D3 agonist molecules with a capacity to bind to iron. Binding assays were carried out with HEK-293 cells expressing either D2 or D3 receptor with tritiated spiperone to evaluate inhibition constants (K(i)). Functional activity of selected compounds was carried out with GTPgammaS binding assay. SAR results identified compounds (+)-19a and (-)-19b as two potent agonists for both D2 and D3 receptors (EC(50) (GTPgammaS); D2 = 4.51 and 1.69 nM and D3 = 1.58 and 0.74 nM for (-)-19b and (+)-19a, respectively). In vitro complexation studies with 19b demonstrated efficient chelation with iron. Furthermore, the deoxyribose assay with 19b demonstrated potent antioxidant activity. In PD animal model study, (-)-19b exhibited potent in vivo activity in reversing locomotor activity in reserpinized rats and also in producing potent rotational activity in 6-OHDA lesioned rats. This reports initial development of unique lead molecules that might find potential use in symptomatic and neuroprotective treatment of PD.
Figures









Similar articles
-
Development of a Highly Potent D2/D3 Agonist and a Partial Agonist from Structure-Activity Relationship Study of N(6)-(2-(4-(1H-Indol-5-yl)piperazin-1-yl)ethyl)-N(6)-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine Analogues: Implication in the Treatment of Parkinson's Disease.J Med Chem. 2015 Dec 10;58(23):9179-95. doi: 10.1021/acs.jmedchem.5b01031. Epub 2015 Nov 30. J Med Chem. 2015. PMID: 26555041 Free PMC article.
-
Bioisosteric heterocyclic versions of 7-{[2-(4-phenyl-piperazin-1-yl)ethyl]propylamino}-5,6,7,8-tetrahydronaphthalen-2-ol: identification of highly potent and selective agonists for dopamine D3 receptor with potent in vivo activity.J Med Chem. 2008 May 22;51(10):3005-19. doi: 10.1021/jm701524h. Epub 2008 Apr 12. J Med Chem. 2008. PMID: 18410082
-
Development of (S)-N6-(2-(4-(isoquinolin-1-yl)piperazin-1-yl)ethyl)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]-thiazole-2,6-diamine and its analogue as a D3 receptor preferring agonist: potent in vivo activity in Parkinson's disease animal models.J Med Chem. 2010 Feb 11;53(3):1023-37. doi: 10.1021/jm901184n. J Med Chem. 2010. PMID: 20038106 Free PMC article.
-
[Pharmacology of antipsychotics at human dopamine D2 and D3 receptors].Nihon Shinkei Seishin Yakurigaku Zasshi. 2012 Feb;32(1):9-18. Nihon Shinkei Seishin Yakurigaku Zasshi. 2012. PMID: 22568121 Review. Japanese.
-
Advances and challenges in the search for D2 and D3 dopamine receptor-selective compounds.Cell Signal. 2018 Jan;41:75-81. doi: 10.1016/j.cellsig.2017.07.003. Epub 2017 Jul 14. Cell Signal. 2018. PMID: 28716664 Free PMC article. Review.
Cited by
-
On the Chemical and Biological Characteristics of Multifunctional Compounds for the Treatment of Parkinson's Disease.Antioxidants (Basel). 2023 Jan 17;12(2):214. doi: 10.3390/antiox12020214. Antioxidants (Basel). 2023. PMID: 36829773 Free PMC article. Review.
-
Multifunctional D2/D3 agonist D-520 with high in vivo efficacy: modulator of toxicity of alpha-synuclein aggregates.ACS Chem Neurosci. 2014 Aug 20;5(8):700-17. doi: 10.1021/cn500084x. Epub 2014 Jul 9. ACS Chem Neurosci. 2014. PMID: 24960209 Free PMC article.
-
Design and Synthesis of Orally Bioavailable Piperazine Substituted 4(1H)-Quinolones with Potent Antimalarial Activity: Structure-Activity and Structure-Property Relationship Studies.J Med Chem. 2018 Feb 22;61(4):1450-1473. doi: 10.1021/acs.jmedchem.7b00738. Epub 2018 Feb 9. J Med Chem. 2018. PMID: 29215279 Free PMC article.
-
Iron Dysregulation and Inflammagens Related to Oral and Gut Health Are Central to the Development of Parkinson's Disease.Biomolecules. 2020 Dec 29;11(1):30. doi: 10.3390/biom11010030. Biomolecules. 2020. PMID: 33383805 Free PMC article. Review.
-
Nitric Oxide, Iron and Neurodegeneration.Front Neurosci. 2019 Feb 18;13:114. doi: 10.3389/fnins.2019.00114. eCollection 2019. Front Neurosci. 2019. PMID: 30833886 Free PMC article. Review.
References
-
- Wooten GF. Movement Disorders. Neurologic Principles and Practice. New York, NY, USA: McGraw-Hill; 1997. pp. 153–160.
-
- Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson's disease. J Neuropathol Exp Neurol. 1991;50:743–755. - PubMed
-
- Mouradian MM. Recent advances in the genetics and pathogenesis of Parkinson disease. Neurology. 2002;58:179–185. - PubMed
-
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. - PubMed
-
- Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53 Suppl 3:S26–S36. discussion S36–S38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

