The tuberous sclerosis complex
- PMID: 20146692
- PMCID: PMC2892799
- DOI: 10.1111/j.1749-6632.2009.05117.x
The tuberous sclerosis complex
Abstract
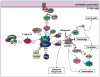
Tuberous sclerosis complex (TSC) is an autosomal dominant disorder that results from mutations in the TSC1 or TSC2 genes and is associated with hamartoma formation in multiple organ systems. The neurological manifestations of TSC are particularly challenging and include infantile spasms, intractable epilepsy, cognitive disabilities, and autism. Progress over the past 15 years has demonstrated that the TSC1 or TSC2 encoded proteins modulate cell function via the mTOR signaling cascade and serve as keystones in regulating cell growth and proliferation. The mTOR pathway provides an intersection for an intricate network of protein cascades that respond to cellular nutrition, energy levels, and growth-factor stimulation. In the brain, TSC1 and TSC2 have been implicated in cell body size, dendritic arborization, axonal outgrowth and targeting, neuronal migration, cortical lamination, and spine formation. Antagonism of the mTOR pathway with rapamycin and related compounds may provide new therapeutic options for TSC patients.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Krsek P, et al. Different features of histopathological subtypes of pediatric focal cortical dysplasia. Ann Neurol. 2008;63:758–769. - PubMed
-
- Mischel PS, et al. Cerebral cortical dysplasia associated with pediatric epilepsy. Review of neuropathologic features and proposal for a grading system. J Neuropathol Exp Neurol. 1995;54:137–153. - PubMed
-
- Andermann F. Cortical dysplasias and epilepsy: a review of the architectonic, clinical, and seizure patterns. Adv Neurol. 2000;84:479–496. - PubMed
-
- Sarnat HB, Flores-Sarnat L. A new classification of malformations of the nervous system: an integration of morphological and molecular genetic criteria as patterns of genetic expression. Eur J Paediatr Neurol. 2001;5:57–64. - PubMed
-
- Palmini A, et al. Terminology and classification of the cortical dysplasias. Neurology. 2004;62:S2–S8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

