Memory CD8+ T cell differentiation
- PMID: 20146720
- PMCID: PMC2836783
- DOI: 10.1111/j.1749-6632.2009.05126.x
Memory CD8+ T cell differentiation
Abstract
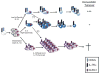
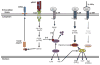
In response to infection or effective vaccination, naive antigen-specific CD8+ T cells undergo a dramatic highly orchestrated activation process. Initial encounter with an appropriately activated antigen-presenting cell leads to blastogenesis and an exponential increase in antigen-specific CD8+ T cell numbers. Simultaneously, a dynamic differentiation process occurs, resulting in formation of both primary effector and long-lived memory cells. Current findings have emphasized the heterogeneity of effector and memory cell populations with the description of multiple cellular subsets based on phenotype, function, and anatomic location. Yet, only recently have we begun to dissect the underlying factors mediating the temporal control of the development of distinct effector and memory CD8+ T cell sublineages. In this review we will focus on the requirements for mounting an effective CD8+ T cell response and highlight the elements regulating the differentiation of effector and memory subsets.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. - PubMed
-
- Nabel GJ. Mapping the future of HIV vaccines. Nat Rev Microbiol. 2007;5:482–484. - PubMed
-
- Todryk SM, Hill AV. Malaria vaccines: the stage we are at. Nat Rev Microbiol. 2007;5:487–489. - PubMed
-
- Hikono H, et al. T-cell memory and recall responses to respiratory virus infections. Immunol Rev. 2006;211:119–132. - PubMed
-
- Hill AV. Pre-erythrocytic malaria vaccines: towards greater efficacy. Nat Rev Immunol. 2006;6:21–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

