A metabolic signature of long life in Caenorhabditis elegans
- PMID: 20146810
- PMCID: PMC2829508
- DOI: 10.1186/1741-7007-8-14
A metabolic signature of long life in Caenorhabditis elegans
Abstract
Background: Many Caenorhabditis elegans mutations increase longevity and much evidence suggests that they do so at least partly via changes in metabolism. However, up until now there has been no systematic investigation of how the metabolic networks of long-lived mutants differ from those of normal worms. Metabolomic technologies, that permit the analysis of many untargeted metabolites in parallel, now make this possible. Here we use one of these, 1H nuclear magnetic resonance spectroscopy, to investigate what makes long-lived worms metabolically distinctive.
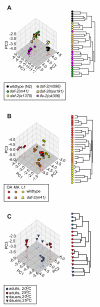
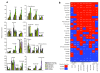
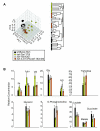
Results: We examined three classes of long-lived worms: dauer larvae, adult Insulin/IGF-1 signalling (IIS)-defective mutants, and a translation-defective mutant. Surprisingly, these ostensibly different long-lived worms share a common metabolic signature, dominated by shifts in carbohydrate and amino acid metabolism. In addition the dauer larvae, uniquely, had elevated levels of modified amino acids (hydroxyproline and phosphoserine). We interrogated existing gene expression data in order to integrate functional (metabolite-level) changes with transcriptional changes at a pathway level.
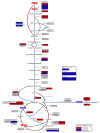
Conclusions: The observed metabolic responses could be explained to a large degree by upregulation of gluconeogenesis and the glyoxylate shunt as well as changes in amino acid catabolism. These responses point to new possible mechanisms of longevity assurance in worms. The metabolic changes observed in dauer larvae can be explained by the existence of high levels of autophagy leading to recycling of cellular components.See associated minireview: http://jbiol.com/content/9/1/7.
Figures

Similar articles
-
Untangling Longevity, Dauer, and Healthspan in Caenorhabditis elegans Insulin/IGF-1-Signalling.Gerontology. 2018;64(1):96-104. doi: 10.1159/000480504. Epub 2017 Sep 22. Gerontology. 2018. PMID: 28934747 Free PMC article.
-
TATN-1 mutations reveal a novel role for tyrosine as a metabolic signal that influences developmental decisions and longevity in Caenorhabditis elegans.PLoS Genet. 2013;9(12):e1004020. doi: 10.1371/journal.pgen.1004020. Epub 2013 Dec 19. PLoS Genet. 2013. PMID: 24385923 Free PMC article.
-
Erratum to "Diapause-associated metabolic traits reiterated in long-lived daf-2 mutants in the nematode Caenorhabditis elegans" [Mech. Ageing Dev. 127 (5) (2006) 458-472].Mech Ageing Dev. 2006 Dec;127(12):922-36. doi: 10.1016/j.mad.2006.10.002. Mech Ageing Dev. 2006. PMID: 17216712
-
Alternate metabolism during the dauer stage of the nematode Caenorhabditis elegans.Exp Gerontol. 2005 Nov;40(11):850-6. doi: 10.1016/j.exger.2005.09.006. Epub 2005 Oct 10. Exp Gerontol. 2005. PMID: 16221538 Review.
-
My adventures with genes from the fountain of youth.Harvey Lect. 2004-2005;100:29-70. Harvey Lect. 2004. PMID: 16970174 Review. No abstract available.
Cited by
-
Comparison of proteomic and metabolomic profiles of mutants of the mitochondrial respiratory chain in Caenorhabditis elegans.Mitochondrion. 2015 Jan;20:95-102. doi: 10.1016/j.mito.2014.12.004. Epub 2014 Dec 19. Mitochondrion. 2015. PMID: 25530493 Free PMC article.
-
FOXO/DAF-16 Activation Slows Down Turnover of the Majority of Proteins in C. elegans.Cell Rep. 2016 Sep 13;16(11):3028-3040. doi: 10.1016/j.celrep.2016.07.088. Cell Rep. 2016. PMID: 27626670 Free PMC article.
-
Regulation of metabolism in Caenorhabditis elegans longevity.J Biol. 2010;9(1):7. doi: 10.1186/jbiol215. Epub 2010 Feb 10. J Biol. 2010. PMID: 20156326 Free PMC article.
-
Disruption of insulin signalling preserves bioenergetic competence of mitochondria in ageing Caenorhabditis elegans.BMC Biol. 2010 Jun 28;8:91. doi: 10.1186/1741-7007-8-91. BMC Biol. 2010. PMID: 20584279 Free PMC article.
-
A genetic model of methionine restriction extends Drosophila health- and lifespan.Proc Natl Acad Sci U S A. 2021 Oct 5;118(40):e2110387118. doi: 10.1073/pnas.2110387118. Proc Natl Acad Sci U S A. 2021. PMID: 34588310 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

