Making a difference together: reciprocal interactions in C. elegans and zebrafish asymmetric neural development
- PMID: 20147373
- PMCID: PMC2827681
- DOI: 10.1242/dev.038695
Making a difference together: reciprocal interactions in C. elegans and zebrafish asymmetric neural development
Abstract
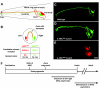
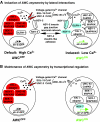
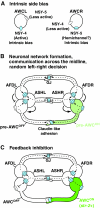
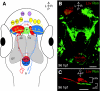
Brain asymmetries are thought to increase neural processing capacity and to prevent interhemispheric conflict. In order to develop asymmetrically, neurons must be specified along the left-right axis, assigned left-side versus right-side identities and differentiate appropriately. In C. elegans and zebrafish, the cellular and molecular mechanisms that lead to neural asymmetries have recently come to light. Here, we consider recent insights into the mechanisms involved in asymmetrical neural development in these two species. Although the molecular details are divergent, both organisms use iterative cell-cell communication to establish left-right neuronal identity.
Figures
Similar articles
-
Gap junctions provide new links in left-right patterning.Cell. 2007 May 18;129(4):645-7. doi: 10.1016/j.cell.2007.05.005. Cell. 2007. PMID: 17512395 Review.
-
Left-right asymmetry: making the right decision early.Curr Biol. 2006 Dec 19;16(24):R1039-42. doi: 10.1016/j.cub.2006.11.009. Curr Biol. 2006. PMID: 17174913
-
Stochastic left-right neuronal asymmetry in Caenorhabditis elegans.Philos Trans R Soc Lond B Biol Sci. 2016 Dec 19;371(1710):20150407. doi: 10.1098/rstb.2015.0407. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27821536 Free PMC article. Review.
-
Asymmetric neural development in the Caenorhabditis elegans olfactory system.Genesis. 2014 Jun;52(6):544-54. doi: 10.1002/dvg.22744. Epub 2014 Feb 7. Genesis. 2014. PMID: 24478264 Free PMC article. Review.
-
An innexin-dependent cell network establishes left-right neuronal asymmetry in C. elegans.Cell. 2007 May 18;129(4):787-99. doi: 10.1016/j.cell.2007.02.052. Cell. 2007. PMID: 17512411
Cited by
-
Intercellular calcium signaling in a gap junction-coupled cell network establishes asymmetric neuronal fates in C. elegans.Development. 2012 Nov;139(22):4191-201. doi: 10.1242/dev.083428. Development. 2012. PMID: 23093425 Free PMC article.
-
FGF signaling is required for brain left-right asymmetry and brain midline formation.Dev Biol. 2014 Feb 1;386(1):123-34. doi: 10.1016/j.ydbio.2013.11.020. Epub 2013 Dec 12. Dev Biol. 2014. PMID: 24333178 Free PMC article.
-
Microtubule-based localization of a synaptic calcium-signaling complex is required for left-right neuronal asymmetry in C. elegans.Development. 2011 Aug;138(16):3509-18. doi: 10.1242/dev.069740. Epub 2011 Jul 19. Development. 2011. PMID: 21771813 Free PMC article.
-
Embryonic Ethanol Exposure Affects the Early Development, Migration, and Location of Hypocretin/Orexin Neurons in Zebrafish.Alcohol Clin Exp Res. 2019 Aug;43(8):1702-1713. doi: 10.1111/acer.14126. Epub 2019 Jul 6. Alcohol Clin Exp Res. 2019. PMID: 31206717 Free PMC article.
-
Fgf signaling governs cell fate in the zebrafish pineal complex.Development. 2013 Jan 15;140(2):323-32. doi: 10.1242/dev.083709. Development. 2013. PMID: 23250206 Free PMC article.
References
-
- Ahmad N., Long S., Rebagliati M. (2004). A southpaw joins the roster: the role of the zebrafish nodal-related gene southpaw in cardiac LR asymmetry. Trends Cardiovasc. Med. 14, 43-49 - PubMed
-
- Aizawa H., Goto M., Sato T., Okamoto H. (2007). Temporally regulated asymmetric neurogenesis causes left-right difference in the zebrafish habenular structures. Dev. Cell 12, 87-98 - PubMed
-
- Amunts K., Schlaug G., Schleicher A., Steinmetz H., Dabringhaus A., Roland P. E., Zilles K. (1996). Asymmetry in the human motor cortex and handedness. Neuroimage 4, 216-222 - PubMed
-
- Asai T., Sugimori E., Tanno Y. (2009). Schizotypal personality traits and atypical lateralization in motor and language functions. Brain Cogn. 71, 26-37 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

