Isolation and propagation of enteric neural crest progenitor cells from mouse embryonic stem cells and embryos
- PMID: 20147374
- PMCID: PMC2827682
- DOI: 10.1242/dev.046896
Isolation and propagation of enteric neural crest progenitor cells from mouse embryonic stem cells and embryos
Abstract
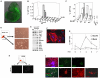
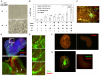
Neural crest is a source of diverse cell types, including the peripheral nervous system. The transcription factor Sox10 is expressed throughout early neural crest. We exploited Sox10 reporter and selection markers created by homologous recombination to investigate the generation, maintenance and expansion of neural crest progenitors. Sox10-GFP-positive cells are produced transiently from mouse embryonic stem (ES) cells by treatment with retinoic acid in combination with Fgf8b and the cytokine leukaemia inhibitory factor (Lif). We found that expression of Sox10 can be maintained using noggin, Wnt3a, Lif and endothelin (NWLE). ES cell-derived Sox10-GFP-positive cells cultured in NWLE exhibit molecular markers of neural crest progenitors. They differentiate into peripheral neurons in vitro and are able to colonise the enteric network in organotypic gut cultures. Neural crest cells purified from embryos using the Sox10 reporter also survive in NWLE, but progressively succumb to differentiation. We therefore applied selection to eliminate differentiating cells. Sox10-selected cells could be clonally expanded, cryopreserved, and multiplied for over 50 days in adherent culture. They remained neurogenic in vitro and in foetal gut grafts. Generation of neural crest from mouse ES cells opens a new route to the identification and validation of determination factors. Furthermore, the ability to propagate undifferentiated progenitors creates an opportunity for experimental dissection of the stimuli and molecular circu that govern neural crest lineage progression. Finally, the demonstration of robust enteric neurogenesis provides a system for investigating and modelling cell therapeutic approaches to neurocristopathies such as Hirschsprung's disease.
Figures



Similar articles
-
Notch signaling is required for the maintenance of enteric neural crest progenitors.Development. 2008 Nov;135(21):3555-65. doi: 10.1242/dev.022319. Epub 2008 Oct 2. Development. 2008. PMID: 18832397
-
L1cam acts as a modifier gene during enteric nervous system development.Neurobiol Dis. 2010 Dec;40(3):622-33. doi: 10.1016/j.nbd.2010.08.006. Epub 2010 Aug 7. Neurobiol Dis. 2010. PMID: 20696247
-
Direct Interaction of Sox10 With Cadherin-19 Mediates Early Sacral Neural Crest Cell Migration: Implications for Enteric Nervous System Development Defects.Gastroenterology. 2022 Jan;162(1):179-192.e11. doi: 10.1053/j.gastro.2021.08.029. Epub 2021 Aug 21. Gastroenterology. 2022. PMID: 34425092
-
The role of SOX10 during enteric nervous system development.Dev Biol. 2013 Oct 1;382(1):330-43. doi: 10.1016/j.ydbio.2013.04.024. Epub 2013 May 2. Dev Biol. 2013. PMID: 23644063 Review.
-
[Phenotypic plasticity of neural crest-derived melanocytes and Schwann cells].Biol Aujourdhui. 2011;205(1):53-61. doi: 10.1051/jbio/2011008. Epub 2011 Apr 19. Biol Aujourdhui. 2011. PMID: 21501576 Review. French.
Cited by
-
Programmed cell death in stem cell-based therapy: Mechanisms and clinical applications.World J Stem Cells. 2021 May 26;13(5):386-415. doi: 10.4252/wjsc.v13.i5.386. World J Stem Cells. 2021. PMID: 34136072 Free PMC article. Review.
-
Generation of murine sympathoadrenergic progenitor-like cells from embryonic stem cells and postnatal adrenal glands.PLoS One. 2013 May 10;8(5):e64454. doi: 10.1371/journal.pone.0064454. Print 2013. PLoS One. 2013. PMID: 23675538 Free PMC article.
-
Deconstructing pancreas development to reconstruct human islets from pluripotent stem cells.Cell Stem Cell. 2010 Apr 2;6(4):300-308. doi: 10.1016/j.stem.2010.03.003. Cell Stem Cell. 2010. PMID: 20362535 Free PMC article.
-
Neural crest stem cells: discovery, properties and potential for therapy.Cell Res. 2012 Feb;22(2):288-304. doi: 10.1038/cr.2012.11. Epub 2012 Jan 10. Cell Res. 2012. PMID: 22231630 Free PMC article. Review.
-
Characterization and transplantation of enteric neural crest cells from human induced pluripotent stem cells.Mol Psychiatry. 2018 Mar;23(3):499-508. doi: 10.1038/mp.2016.191. Epub 2016 Oct 25. Mol Psychiatry. 2018. PMID: 27777423 Free PMC article.
References
-
- Aubert J., Dunstan H., Chambers I., Smith A. (2002). Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat. Biotechnol. 20, 1240-1245 - PubMed
-
- Bain G., Kitchens D., Yao M., Huettner J. E., Gottlieb D. I. (1995). Embryonic stem cells express neuronal properties in vitro. Dev. Biol. 168, 342-357 - PubMed
-
- Barlow A., de Graaff E., Pachnis V. (2003). Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron 40, 905-916 - PubMed
-
- Barton M., Yanagisawa M. (2008). Endothelin: 20 years from discovery to therapy. Can. J. Physiol. Pharmacol. 86, 485-498 - PubMed
-
- Basch M. L., Bronner-Fraser M., Garcia-Castro M. I. (2006). Specification of the neural crest occurs during gastrulation and requires Pax7. Nature 441, 218-222 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

