COUP-TFs regulate eye development by controlling factors essential for optic vesicle morphogenesis
- PMID: 20147377
- PMCID: PMC2827684
- DOI: 10.1242/dev.040568
COUP-TFs regulate eye development by controlling factors essential for optic vesicle morphogenesis
Abstract
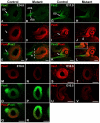
Transcriptional networks, which are initiated by secreted proteins, cooperate with each other to orchestrate eye development. The establishment of dorsal/ventral polarity, especially dorsal specification in the optic vesicle, is poorly understood at a molecular and cellular level. Here, we show that COUP-TFI (Nr2f1) and COUP-TFII (Nr2f2) are highly expressed in the progenitor cells in the developing murine eye. Phenotype analysis of COUP-TFI and COUP-TFII single-gene conditional knockout mouse models suggests that COUP-TFs compensate for each other to maintain morphogenesis of the eye. However, in eye-specific COUP-TFI/TFII double-knockout mice, progenitor cells at the dorso-distal optic vesicle fail to differentiate appropriately, causing the retinal pigmented epithelium cells to adopt a neural retina fate and abnormal differentiation of the dorsal optic stalk; the development of proximo-ventral identities, neural retina and ventral optic stalk is also compromised. These cellular defects in turn lead to congenital ocular colobomata and microphthalmia. Immunohistochemical and in situ hybridization assays reveal that the expression of several regulatory genes essential for early optic vesicle development, including Pax6, Otx2, Mitf, Pax2 and Vax1/2, is altered in the corresponding compartments of the mutant eye. Using ChIP assay, siRNA treatment and transient transfection in ARPE-19 cells in vitro, we demonstrate that Pax6 and Otx2 are directly regulated by COUP-TFs. Taken together, our findings reveal novel and distinct cell-intrinsic mechanisms mediated by COUP-TF genes to direct the specification and differentiation of progenitor cells, and that COUP-TFs are crucial for dorsalization of the eye.
Figures






References
-
- Azuma N., Tadokoro K., Asaka A., Yamada M., Yamaguchi Y., Handa H., Matsushima S., Watanabe T., Kida Y., Ogura T., et al. (2005). Transdifferentiation of the retinal pigment epithelia to the neural retina by transfer of the Pax6 transcriptional factor. Hum. Mol. Genet. 14, 1059-1068 - PubMed
-
- Baumer N., Marquardt T., Stoykova A., Spieler D., Treichel D., Ashery-Padan R., Gruss P. (2003). Retinal pigmented epithelium determination requires the redundant activities of Pax2 and Pax6. Development 130, 2903-2915 - PubMed
-
- Bramblett D., Pennesi M., Wu S., Tsai M. (2004). The transcription factor Bhlhb4 is required for rod bipolar cell maturation. Neuron 43, 779-793 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

