Cadherin-7 and cadherin-6B differentially regulate the growth, branching and guidance of cranial motor axons
- PMID: 20147381
- PMCID: PMC2827690
- DOI: 10.1242/dev.042457
Cadherin-7 and cadherin-6B differentially regulate the growth, branching and guidance of cranial motor axons
Abstract
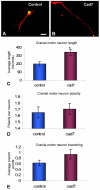
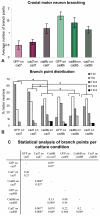
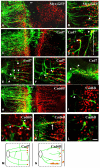
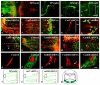
Cadherin-7 (Cad7) and cadherin-6B (Cad6B) are expressed in early and late phases of cranial motoneuron development, respectively. Cad7 is expressed by cranial motoneurons soon after they are generated, as well as in the environment through which their axons extend. By contrast, Cad6B is expressed by mature cranial motoneurons. We demonstrate in chick that these cadherins play distinct roles in cranial motor axon morphology, branching and projection. Using in vitro approaches, we show that Cad7 enhances motor axon outgrowth, suppresses the formation of multiple axons and restricts interstitial branching, thus promoting the development of a single unbranched axon characteristic of differentiating motoneurons. Conversely, Cad6B in vitro promotes motor axon branching, a characteristic of mature motoneurons. In vivo gain- and loss-of-function experiments for these cadherins yielded phenotypes consistent with this interpretation. In particular, a loss of cadherin-mediated interactions in vivo led to dysregulation of the cranial motoneuron normal branching programme and caused axon navigation defects. We also demonstrate that Cad6B functions via the phosphatidylinositol 3-kinase pathway. Together, these data show that Cad7 and Cad6B differentially regulate cranial motoneuron growth, branching and axon guidance.
Figures



Similar articles
-
Cadherin-6B proteolytic N-terminal fragments promote chick cranial neural crest cell delamination by regulating extracellular matrix degradation.Dev Biol. 2018 Dec 1;444 Suppl 1(Suppl 1):S237-S251. doi: 10.1016/j.ydbio.2018.06.018. Epub 2018 Jun 27. Dev Biol. 2018. PMID: 29958899 Free PMC article.
-
Tetraspanin18 is a FoxD3-responsive antagonist of cranial neural crest epithelial-to-mesenchymal transition that maintains cadherin-6B protein.J Cell Sci. 2013 Mar 15;126(Pt 6):1464-76. doi: 10.1242/jcs.120915. Epub 2013 Feb 15. J Cell Sci. 2013. PMID: 23418345 Free PMC article.
-
Central topography of cranial motor nuclei controlled by differential cadherin expression.Curr Biol. 2014 Nov 3;24(21):2541-7. doi: 10.1016/j.cub.2014.08.067. Epub 2014 Oct 9. Curr Biol. 2014. PMID: 25308074 Free PMC article.
-
Patterning and axon guidance of cranial motor neurons.Nat Rev Neurosci. 2007 Nov;8(11):859-71. doi: 10.1038/nrn2254. Nat Rev Neurosci. 2007. PMID: 17948031 Review.
-
Axons get ahead: Insights into axon guidance and congenital cranial dysinnervation disorders.Dev Neurobiol. 2017 Jul;77(7):861-875. doi: 10.1002/dneu.22477. Epub 2017 May 22. Dev Neurobiol. 2017. PMID: 28033651 Review.
Cited by
-
Cadherin-based transsynaptic networks in establishing and modifying neural connectivity.Curr Top Dev Biol. 2015;112:415-65. doi: 10.1016/bs.ctdb.2014.11.025. Epub 2015 Feb 11. Curr Top Dev Biol. 2015. PMID: 25733148 Free PMC article. Review.
-
Cadherin 8 regulates proliferation of cortical interneuron progenitors.Brain Struct Funct. 2019 Jan;224(1):277-292. doi: 10.1007/s00429-018-1772-4. Epub 2018 Oct 12. Brain Struct Funct. 2019. PMID: 30315415 Free PMC article.
-
Intrinsic properties guide proximal abducens and oculomotor nerve outgrowth in avian embryos.Dev Neurobiol. 2012 Feb;72(2):167-85. doi: 10.1002/dneu.20948. Dev Neurobiol. 2012. PMID: 21739615 Free PMC article.
-
Chicken embryo spinal cord slice culture protocol.J Vis Exp. 2013 Mar 25;(73):50295. doi: 10.3791/50295. J Vis Exp. 2013. PMID: 23568251 Free PMC article.
-
Catenin-dependent cadherin function drives divisional segregation of spinal motor neurons.J Neurosci. 2012 Jan 11;32(2):490-505. doi: 10.1523/JNEUROSCI.4382-11.2012. J Neurosci. 2012. PMID: 22238085 Free PMC article.
References
-
- Boscher C., Mege R. M. (2008). Cadherin-11 interacts with the FGF receptor and induces neurite outgrowth through associated downstream signalling. Cell Signal. 20, 1061-1072 - PubMed
-
- Calautti E., Li J., Saoncella S., Brissette J. L., Goetinck P. F. (2005). Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J. Biol. Chem. 280, 32856-32865 - PubMed
-
- Caton A., Hacker A., Naeem A., Livet J., Maina F., Bladt F., Klein R., Birchmeier C., Guthrie S. (2000). The branchial arches and HGF are growth-promoting and chemoattractant for cranial motor axons. Development 127, 1751-1766 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

