Simian virus 40 T/t antigens and lamin A/C small interfering RNA rescue the phenotype of an Epstein-Barr virus protein kinase (BGLF4) mutant
- PMID: 20147387
- PMCID: PMC2863785
- DOI: 10.1128/JVI.02456-09
Simian virus 40 T/t antigens and lamin A/C small interfering RNA rescue the phenotype of an Epstein-Barr virus protein kinase (BGLF4) mutant
Abstract
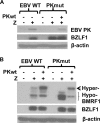
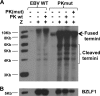
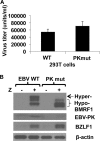
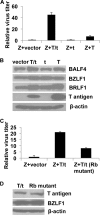
The Epstein-Barr virus (EBV)-encoded viral protein kinase, EBV-PK (the BGLF4 gene product), is required for efficient nuclear viral egress in 293 cells. However, since EBV-PK phosphorylates a number of different viral and cellular proteins (including lamin A/C), the relative importance of each target during lytic viral replication remains unclear. We show here that an EBV PK mutant (PKmut; containing stop codons at residues 1 and 5 in EBV-PK) is highly defective for release of infectious virus from 293 cells but not 293T cells. Furthermore, the phenotype of the PKmut in 293 cells is substantially reversed by expression of the simian virus 40 (SV40) large (T) and small (t) T antigens. Efficient rescue requires the presence of both SV40 T/t proteins. We show that 293T cells have a much higher level of constitutive lamin A/C phosphorylation than do 293 cells over residues (S22 and S392) that promote phosphorylation-dependent nuclear disassembly and that both large T and small t contribute to enhanced lamin A/C phosphorylation. Finally, we demonstrate that knockdown of lamin A/C expression using small interfering RNA also rescues the PKmut phenotype in 293 cells. These results suggest that essential roles of EBV-PK during lytic viral replication include the phosphorylation and dispersion of lamin A/C.
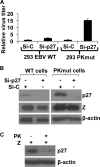
Figures





Similar articles
-
The Epstein-Barr virus (EBV)-encoded protein kinase, EBV-PK, but not the thymidine kinase (EBV-TK), is required for ganciclovir and acyclovir inhibition of lytic viral production.J Virol. 2010 May;84(9):4534-42. doi: 10.1128/JVI.02487-09. Epub 2010 Feb 24. J Virol. 2010. PMID: 20181711 Free PMC article.
-
Epstein-Barr virus BGLF4 kinase induces disassembly of the nuclear lamina to facilitate virion production.J Virol. 2008 Dec;82(23):11913-26. doi: 10.1128/JVI.01100-08. Epub 2008 Sep 24. J Virol. 2008. PMID: 18815303 Free PMC article.
-
Epstein-Barr virus-encoded protein kinase (BGLF4) is involved in production of infectious virus.J Virol. 2007 May;81(10):5407-12. doi: 10.1128/JVI.02398-06. Epub 2007 Mar 14. J Virol. 2007. PMID: 17360761 Free PMC article.
-
Conquering the Nuclear Envelope Barriers by EBV Lytic Replication.Viruses. 2021 Apr 18;13(4):702. doi: 10.3390/v13040702. Viruses. 2021. PMID: 33919628 Free PMC article. Review.
-
Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases.Autoimmunity. 2008 May;41(4):298-328. doi: 10.1080/08916930802024772. Autoimmunity. 2008. PMID: 18432410 Review.
Cited by
-
The Epstein-Barr virus (EBV)-encoded protein kinase, EBV-PK, but not the thymidine kinase (EBV-TK), is required for ganciclovir and acyclovir inhibition of lytic viral production.J Virol. 2010 May;84(9):4534-42. doi: 10.1128/JVI.02487-09. Epub 2010 Feb 24. J Virol. 2010. PMID: 20181711 Free PMC article.
-
Cellular transcription factor Oct-1 interacts with the Epstein-Barr virus BRLF1 protein to promote disruption of viral latency.J Virol. 2011 Sep;85(17):8940-53. doi: 10.1128/JVI.00569-11. Epub 2011 Jun 22. J Virol. 2011. PMID: 21697476 Free PMC article.
-
Key motifs in EBV (Epstein-Barr virus)-encoded protein kinase for phosphorylation activity and nuclear localization.Biochem J. 2010 Oct 15;431(2):227-35. doi: 10.1042/BJ20100558. Biochem J. 2010. PMID: 20704565 Free PMC article.
-
Expression of the Conserved Herpesvirus Protein Kinase (CHPK) of Marek's Disease Alphaherpesvirus in the Skin Reveals a Mechanistic Importance for CHPK during Interindividual Spread in Chickens.J Virol. 2020 Feb 14;94(5):e01522-19. doi: 10.1128/JVI.01522-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31801854 Free PMC article.
-
The Conserved Herpesviridae Protein Kinase (CHPK) of Gallid alphaherpesvirus 3 (GaHV3) Is Required for Horizontal Spread and Natural Infection in Chickens.Viruses. 2022 Mar 12;14(3):586. doi: 10.3390/v14030586. Viruses. 2022. PMID: 35336996 Free PMC article.
References
-
- Aebi, U., J. Cohn, L. Buhle, and L. Gerace. 1986. The nuclear lamina is a meshwork of intermediate-type filaments. Nature 323:560-564. - PubMed
-
- Arroyo, J. D., and W. C. Hahn. 2005. Involvement of PP2A in viral and cellular transformation. Oncogene 24:7746-7755. - PubMed
-
- Asai, R., A. Kato, K. Kato, M. Kanamori-Koyama, K. Sugimoto, T. Sairenji, Y. Nishiyama, and Y. Kawaguchi. 2006. Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1. J. Virol. 80:5125-5134. - PMC - PubMed
-
- Asai, R., A. Kato, and Y. Kawaguchi. 2009. Epstein-Barr virus protein kinase BGLF4 interacts with viral transactivator BZLF1 and regulates its transactivation activity. J. Gen. Virol. 90:1575-1581. - PubMed
-
- Chang, L. S., M. M. Pater, N. I. Hutchinson, and G. di Mayorca. 1984. Transformation by purified early genes of simian virus 40. Virology 133:341-353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA019014/CA/NCI NIH HHS/United States
- R01 CA066519/CA/NCI NIH HHS/United States
- T32 AI078985/AI/NIAID NIH HHS/United States
- R01-H6064851/PHS HHS/United States
- R01-CA66519/CA/NCI NIH HHS/United States
- P01 CA022443/CA/NCI NIH HHS/United States
- P01-CA019014/CA/NCI NIH HHS/United States
- R01 CA058853/CA/NCI NIH HHS/United States
- R01 AI074984/AI/NIAID NIH HHS/United States
- P01-CA022443/CA/NCI NIH HHS/United States
- R01-AI074984/AI/NIAID NIH HHS/United States
- R01-CA58853/CA/NCI NIH HHS/United States
- T32-AI078985/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

