CD147/EMMPRIN acts as a functional entry receptor for measles virus on epithelial cells
- PMID: 20147391
- PMCID: PMC2863760
- DOI: 10.1128/JVI.02168-09
CD147/EMMPRIN acts as a functional entry receptor for measles virus on epithelial cells
Abstract
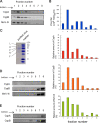
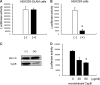
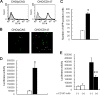
Measles is a highly contagious human disease caused by measles virus (MeV) and remains the leading cause of death in children, particularly in developing countries. Wild-type MeV preferentially infects lymphocytes by using signaling lymphocytic activation molecule (SLAM), whose expression is restricted to hematopoietic cells, as a receptor. MeV also infects other epithelial and neuronal cells that do not express SLAM and causes pneumonia and diarrhea and, sometimes, serious symptoms such as measles encephalitis and subacute sclerosing panencephalitis. The discrepancy between the tissue tropism of MeV and the distribution of SLAM-positive cells suggests that there are unknown receptors other than SLAM for MeV. Here we identified CD147/EMMPRIN (extracellular matrix metalloproteinase inducer), a transmembrane glycoprotein, which acts as a receptor for MeV on epithelial cells. Furthermore, we found the incorporation of cyclophilin B (CypB), a cellular ligand for CD147, in MeV virions, and showed that inhibition of CypB incorporation significantly attenuated SLAM-independent infection on epithelial cells, while it had no effect on SLAM-dependent infection. To date, MeV infection was considered to be triggered by binding of its hemagglutinin (H) protein and cellular receptors. Our present study, however, indicates that MeV infection also occurs via CD147 and virion-associated CypB, independently of MeV H. Since CD147 is expressed in a variety of cells, including epithelial and neuronal cells, this molecule possibly functions as an entry receptor for MeV in SLAM-negative cells. This is the first report among members of the Mononegavirales that CD147 is used as a virus entry receptor via incorporated CypB in the virions.
Figures




Similar articles
-
Measles Virus Enters Breast and Colon Cancer Cell Lines through a PVRL4-Mediated Macropinocytosis Pathway.J Virol. 2017 Apr 28;91(10):e02191-16. doi: 10.1128/JVI.02191-16. Print 2017 May 15. J Virol. 2017. PMID: 28250131 Free PMC article.
-
Interaction of the Hemagglutinin Stalk Region with Cell Adhesion Molecule (CADM) 1 and CADM2 Mediates the Spread between Neurons and Neuropathogenicity of Measles Virus with a Hyperfusogenic Fusion Protein.J Virol. 2023 May 31;97(5):e0034023. doi: 10.1128/jvi.00340-23. Epub 2023 May 11. J Virol. 2023. PMID: 37166307 Free PMC article.
-
Weak cis and trans Interactions of the Hemagglutinin with Receptors Trigger Fusion Proteins of Neuropathogenic Measles Virus Isolates.J Virol. 2020 Jan 6;94(2):e01727-19. doi: 10.1128/JVI.01727-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31619560 Free PMC article.
-
Structural characteristics of measles virus entry.Curr Opin Virol. 2020 Apr;41:52-58. doi: 10.1016/j.coviro.2020.04.002. Epub 2020 May 12. Curr Opin Virol. 2020. PMID: 32413678 Review.
-
Measles virus receptors.Curr Top Microbiol Immunol. 2009;329:13-30. doi: 10.1007/978-3-540-70523-9_2. Curr Top Microbiol Immunol. 2009. PMID: 19198560 Review.
Cited by
-
Protective vascular coagulation in response to bacterial infection of the kidney is regulated by bacterial lipid A and host CD147.Pathog Dis. 2018 Nov 1;76(8):fty087. doi: 10.1093/femspd/fty087. Pathog Dis. 2018. PMID: 30476069 Free PMC article.
-
Ehrlichia chaffeensis Uses an Invasin To Suppress Reactive Oxygen Species Generation by Macrophages via CD147-Dependent Inhibition of Vav1 To Block Rac1 Activation.mBio. 2020 Apr 21;11(2):e00267-20. doi: 10.1128/mBio.00267-20. mBio. 2020. PMID: 32317318 Free PMC article.
-
Interaction of stellate cells with pancreatic carcinoma cells.Cancers (Basel). 2010 Sep 9;2(3):1661-82. doi: 10.3390/cancers2031661. Cancers (Basel). 2010. PMID: 24281180 Free PMC article.
-
Cardiorenal Tissues Express SARS-CoV-2 Entry Genes and Basigin (BSG/CD147) Increases With Age in Endothelial Cells.JACC Basic Transl Sci. 2020 Nov;5(11):1111-1123. doi: 10.1016/j.jacbts.2020.09.010. Epub 2020 Oct 9. JACC Basic Transl Sci. 2020. PMID: 33073064 Free PMC article.
-
Spike S1 domain interactome in non-pulmonary systems: A role beyond the receptor recognition.Front Mol Biosci. 2022 Sep 26;9:975570. doi: 10.3389/fmolb.2022.975570. eCollection 2022. Front Mol Biosci. 2022. PMID: 36225252 Free PMC article.
References
-
- Biswas, C., Y. Zhang, R. DeCastro, H. Guo, T. Nakamura, H. Kataoka, and K. Nabeshima. 1995. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 55:434-439. - PubMed
-
- Bose, S., M. Mathur, P. Baltes, N. Joshi, and A. K. Banerjee. 2003. Requirement for cyclophilin A for the replication of vesicular stomatitis virus New Jersey serotype. J. Gen. Virol. 84:1687-1699. - PubMed
-
- Bryce, J., C. Boschi-Pinto, K. Shibuya, and R. E. Black. 2005. WHO estimates of the causes of death in children. Lancet 365:1147-1152. - PubMed
-
- Cande, C., N. Vahsen, I. Kouranti, E. Schmitt, E. Daugas, C. Spahr, J. Luban, R. T. Kroemer, F. Giordanetto, C. Garrido, and J. M. Penninger. 2004. AIF and cyclophilin A cooperate in apoptosis-associated chromatinolysis. Oncogene 23:1514-1521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

