Lentiviral Nef proteins utilize PAK2-mediated deregulation of cofilin as a general strategy to interfere with actin remodeling
- PMID: 20147394
- PMCID: PMC2849517
- DOI: 10.1128/JVI.02467-09
Lentiviral Nef proteins utilize PAK2-mediated deregulation of cofilin as a general strategy to interfere with actin remodeling
Abstract
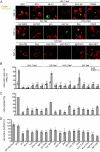
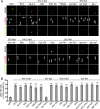
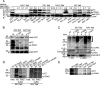
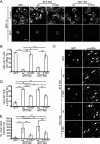
Nef is an accessory protein and pathogenicity factor of human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) which elevates virus replication in vivo. We recently described for HIV type 1(SF2) (HIV-1(SF2)) the potent interference of Nef with T-lymphocyte chemotaxis via its association with the cellular kinase PAK2. Mechanistic analysis revealed that this interaction results in deregulation of the actin-severing factor cofilin and thus blocks the chemokine-mediated actin remodeling required for cell motility. However, the efficiency of PAK2 association is highly variable among Nef proteins from different lentiviruses, prompting us to evaluate the conservation of this actin-remodeling/cofilin-deregulating mechanism. Based on the analysis of a total of 17 HIV-1, HIV-2, and SIV Nef proteins, we report here that inhibition of chemokine-induced actin remodeling as well as inactivation of cofilin are strongly conserved activities of lentiviral Nef proteins. Of note, even for Nef variants that display only marginal PAK2 association in vitro, these activities require the integrity of a PAK2 recruitment motif and the presence of endogenous PAK2. Thus, reduced in vitro affinity to PAK2 does not indicate limited functionality of Nef-PAK2 complexes in intact HIV-1 host cells. These results establish hijacking of PAK2 for deregulation of cofilin and inhibition of triggered actin remodeling as a highly conserved function of lentiviral Nef proteins, supporting the notion that PAK2 association may be critical for Nef's activity in vivo.
Figures



Similar articles
-
Association with PAK2 Enables Functional Interactions of Lentiviral Nef Proteins with the Exocyst Complex.mBio. 2015 Sep 8;6(5):e01309-15. doi: 10.1128/mBio.01309-15. mBio. 2015. PMID: 26350970 Free PMC article.
-
HIV-1 Nef interferes with host cell motility by deregulation of Cofilin.Cell Host Microbe. 2009 Aug 20;6(2):174-86. doi: 10.1016/j.chom.2009.06.004. Cell Host Microbe. 2009. PMID: 19683683
-
Identification of a highly conserved valine-glycine-phenylalanine amino acid triplet required for HIV-1 Nef function.Retrovirology. 2012 Apr 27;9:34. doi: 10.1186/1742-4690-9-34. Retrovirology. 2012. PMID: 22537596 Free PMC article.
-
How HIV Nef Proteins Hijack Membrane Traffic To Promote Infection.J Virol. 2019 Nov 26;93(24):e01322-19. doi: 10.1128/JVI.01322-19. Print 2019 Dec 15. J Virol. 2019. PMID: 31578291 Free PMC article. Review.
-
Human immunodeficiency virus (HIV) type-1, HIV-2 and simian immunodeficiency virus Nef proteins.Mol Aspects Med. 2010 Oct;31(5):418-33. doi: 10.1016/j.mam.2010.05.003. Epub 2010 Jun 4. Mol Aspects Med. 2010. PMID: 20594957 Review.
Cited by
-
Estradiol inhibits HIV-1BaL infection and induces CFL1 expression in peripheral blood mononuclear cells and endocervical mucosa.Sci Rep. 2022 Apr 13;12(1):6165. doi: 10.1038/s41598-022-10163-6. Sci Rep. 2022. PMID: 35418661 Free PMC article.
-
Primate lentiviral Nef proteins deregulate T-cell development by multiple mechanisms.Retrovirology. 2013 Nov 15;10:137. doi: 10.1186/1742-4690-10-137. Retrovirology. 2013. PMID: 24237970 Free PMC article.
-
LIM kinase 1 - dependent cofilin 1 pathway and actin dynamics mediate nuclear retinoid receptor function in T lymphocytes.BMC Mol Biol. 2011 Sep 16;12:41. doi: 10.1186/1471-2199-12-41. BMC Mol Biol. 2011. PMID: 21923909 Free PMC article.
-
HIV-1 Nef interferes with T-lymphocyte circulation through confined environments in vivo.Proc Natl Acad Sci U S A. 2012 Nov 6;109(45):18541-6. doi: 10.1073/pnas.1204322109. Epub 2012 Oct 23. Proc Natl Acad Sci U S A. 2012. PMID: 23093676 Free PMC article.
-
Multifunctional Roles of the N-Terminal Region of HIV-1SF2Nef Are Mediated by Three Independent Protein Interaction Sites.J Virol. 2019 Dec 12;94(1):e01398-19. doi: 10.1128/JVI.01398-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597760 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

