Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques
- PMID: 20147396
- PMCID: PMC2863788
- DOI: 10.1128/JVI.02279-09
Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques
Abstract
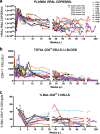
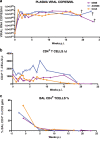
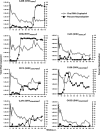
A new pathogenic R5-tropic simian/human immunodeficiency virus (SHIV) was generated following serial passaging in rhesus macaques. All 13 animals inoculated with SHIV(AD8) passaged lineages experienced marked depletions of CD4(+) T cells. Ten of these infected monkeys became normal progressors (NPs) and had gradual losses of both memory and naïve CD4(+) T lymphocytes, generated antiviral CD4(+) and CD8(+) T cell responses, and sustained chronic immune activation while maintaining variable levels of plasma viremia (10(2) to 10(5) RNA copies/ml for up to 3 years postinfection [p.i.]). To date, five NPs developed AIDS associated with opportunistic infections caused by Pneumocystis carinii, Mycobacterium avium, and Campylobacter coli that required euthanasia between weeks 100 and 199 p.i. Three other NPs have experienced marked depletions of circulating CD4(+) T lymphocytes (92 to 154 cells/microl) following 1 to 2 years of infection. When tested for coreceptor usage, the viruses isolated from four NPs at the time of their euthanasia remained R5 tropic. Three of the 13 SHIV(AD8)-inoculated macaques experienced a rapid-progressor syndrome characterized by sustained plasma viremia of >1 x 10(7) RNA copies/ml and rapid irreversible loss of memory CD4(+) T cells that required euthanasia between weeks 19 and 23 postinfection. The sustained viremia, associated depletion of CD4(+) T lymphocytes, and induction of AIDS make the SHIV(AD8) lineage of viruses a potentially valuable reagent for vaccine studies.
Figures
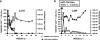
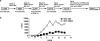
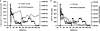
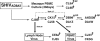
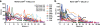

References
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Barnett, S. W., I. K. Srivastava, E. Kan, F. Zhou, A. Goodsell, A. D. Cristillo, M. G. Ferrai, D. E. Weiss, N. L. Letvin, D. Montefiori, R. Pal, and M. Vajdy. 2008. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS 22:339-348. - PubMed
-
- Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. - PubMed
-
- Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

