Type I interferon-sensitive recombinant newcastle disease virus for oncolytic virotherapy
- PMID: 20147405
- PMCID: PMC2849496
- DOI: 10.1128/JVI.01553-09
Type I interferon-sensitive recombinant newcastle disease virus for oncolytic virotherapy
Abstract
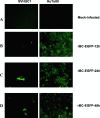
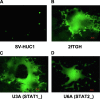
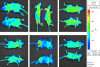
Newcastle disease virus (NDV), an avian paramyxovirus, is tumor selective and intrinsically oncolytic because of its potent ability to induce apoptosis. Several studies have demonstrated that NDV is selectively cytotoxic to tumor cells but not normal cells due to defects in the interferon (IFN) antiviral responses of tumor cells. Many naturally occurring strains of NDV have an intact IFN-antagonistic function and can still replicate in normal human cells. To avoid potential toxicity issues with NDV, especially in cancer patients with immunosuppression, safe NDV-oncolytic vectors are needed. We compared the cell killing abilities of (i) a recombinant NDV (rNDV) strain, Beaudette C, containing an IFN-antagonistic, wild-type V protein (rBC), (ii) an isogenic recombinant virus with a mutant V protein (rBC-Edit virus) that induces increased IFN in infected cells and whose replication is restricted in normal human cells, and (iii) a recombinant LaSota virus with a virulent F protein cleavage site that is as interferon sensitive as rBC-Edit virus (LaSota V.F. virus). Our results indicated that the tumor-selective replication of rNDV is determined by the differential regulation of IFN-alpha and downstream antiviral genes induced by IFN-alpha, especially through the IRF-7 pathway. In a nude mouse model of human fibrosarcoma, we show that the IFN-sensitive NDV variants are as effective as IFN-resistant rBC virus in clearing the tumor burden. In addition, mice treated with rNDV exhibited no signs of toxicity to the viruses. These findings indicate that augmentation of innate immune responses by NDV results in selective oncolysis and offer a novel and safe virotherapy platform.
Figures
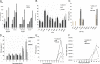


Similar articles
-
Cell-type-specific innate immune response to oncolytic Newcastle disease virus.Viral Immunol. 2012 Aug;25(4):268-76. doi: 10.1089/vim.2012.0020. Epub 2012 Jul 18. Viral Immunol. 2012. PMID: 22808996 Free PMC article.
-
Recombinant Immunomodulating Lentogenic or Mesogenic Oncolytic Newcastle Disease Virus for Treatment of Pancreatic Adenocarcinoma.Viruses. 2015 Jun 11;7(6):2980-98. doi: 10.3390/v7062756. Viruses. 2015. PMID: 26110582 Free PMC article.
-
Genetic Modification of Oncolytic Newcastle Disease Virus for Cancer Therapy.J Virol. 2016 May 12;90(11):5343-5352. doi: 10.1128/JVI.00136-16. Print 2016 Jun 1. J Virol. 2016. PMID: 27009956 Free PMC article.
-
Oncolytic therapy and gene therapy for cancer: recent advances in antitumor effects of Newcastle disease virus.Discov Med. 2020 Jul-Aug;30(159):39-48. Discov Med. 2020. PMID: 33357361 Review.
-
Oncolytic Newcastle disease virus as a prospective anti-cancer therapy. A biologic agent with potential to break therapy resistance.Expert Opin Biol Ther. 2015;15(12):1757-71. doi: 10.1517/14712598.2015.1088000. Epub 2015 Oct 5. Expert Opin Biol Ther. 2015. PMID: 26436571 Review.
Cited by
-
Newcastle disease virus selectively infects dividing cells and promotes viral proliferation.Vet Res. 2019 Apr 18;50(1):27. doi: 10.1186/s13567-019-0644-0. Vet Res. 2019. PMID: 30999941 Free PMC article.
-
Lysis-independent potentiation of immune checkpoint blockade by oncolytic virus.Oncotarget. 2018 Jun 19;9(47):28702-28716. doi: 10.18632/oncotarget.25614. eCollection 2018 Jun 19. Oncotarget. 2018. PMID: 29983890 Free PMC article.
-
Transcriptome Analysis of Natural Killer Cells in Response to Newcastle Disease Virus Infected Hepatocellular Carcinoma Cells.Genes (Basel). 2023 Apr 10;14(4):888. doi: 10.3390/genes14040888. Genes (Basel). 2023. PMID: 37107646 Free PMC article.
-
Prostate-specific antigen-retargeted recombinant newcastle disease virus for prostate cancer virotherapy.J Virol. 2013 Apr;87(7):3792-800. doi: 10.1128/JVI.02394-12. Epub 2013 Jan 23. J Virol. 2013. PMID: 23345509 Free PMC article.
-
Recombinant Newcastle disease virus (rL-RVG) triggers autophagy and apoptosis in gastric carcinoma cells by inducing ER stress.Am J Cancer Res. 2016 May 1;6(5):924-36. eCollection 2016. Am J Cancer Res. 2016. PMID: 27293989 Free PMC article.
References
-
- Advani, A. S., and A. M. Pendergast. 2002. Bcr-Abl variants: biological and clinical aspects. Leukoc. Res. 26:713-720. - PubMed
-
- Arora, T., G. Floyd-Smith, M. J. Espy, and D. F. Jelinek. 1999. Dissociation between IFN-alpha-induced anti-viral and growth signaling pathways. J. Immunol. 162:3289-3297. - PubMed
-
- Bell, S., S. Hansen, and J. Buchner. 2002. Refolding and structural characterization of the human p53 tumor suppressor protein. Biophys. Chem. 96:243-257. - PubMed
-
- Bello, M. J., J. M. de Campos, M. E. Kusak, J. Vaquero, J. L. Sarasa, A. Pestana, and J. A. Rey. 1994. Allelic loss at 1p is associated with tumor progression of meningiomas. Genes Chromosomes Cancer 9:296-298. - PubMed
-
- Bian, H., P. Fournier, B. Peeters, and V. Schirrmacher. 2005. Tumor-targeted gene transfer in vivo via recombinant Newcastle disease virus modified by a bispecific fusion protein. Int. J. Oncol. 27:377-384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

