A novel inhibitor of the NF-{kappa}B signaling pathway encoded by the parapoxvirus orf virus
- PMID: 20147406
- PMCID: PMC2849485
- DOI: 10.1128/JVI.02291-09
A novel inhibitor of the NF-{kappa}B signaling pathway encoded by the parapoxvirus orf virus
Abstract
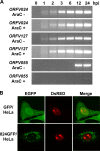
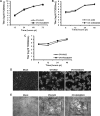
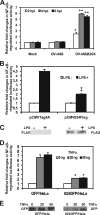
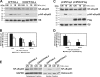
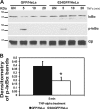
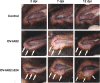
The parapoxvirus orf virus (ORFV) is a pathogen of sheep and goats that has been used as a preventive and therapeutic immunomodulatory agent in several animal species. However, the functions (genes, proteins, and mechanisms of action) evolved by ORFV to modulate and manipulate immune responses are poorly understood. Here, the novel ORFV protein ORFV024 was shown to inhibit activation of the NF-kappaB signaling pathway, an important modulator of early immune responses against viral infections. Infection of primary ovine cells with an ORFV024 deletion mutant virus resulted in a marked increase in expression of NF-kappaB-regulated chemokines and other proinflammatory host genes. Expression of ORFV024 in cell cultures significantly decreased lipopolysaccharide (LPS)- and tumor necrosis factor alpha (TNF-alpha)-induced NF-kappaB-responsive reporter gene expression. Further, ORFV024 expression decreased TNF-alpha-induced phosphorylation and nuclear translocation of NF-kappaB-p65, phosphorylation, and degradation of IkappaBalpha, and phosphorylation of IkappaB kinase (IKK) subunits IKKalpha and IKKbeta, indicating that ORFV024 functions by inhibiting activation of IKKs, the bottleneck for most NF-kappaB activating stimuli. Although ORFV024 interferes with activation of the NF-kappaB signaling pathway, its deletion from the OV-IA82 genome had no significant effect on disease severity, progression, and time to resolution in sheep, indicating that ORFV024 is not essential for virus virulence in the natural host. This represents the first description of a NF-kappaB inhibitor encoded by a parapoxvirus.
Figures

Similar articles
-
A nuclear inhibitor of NF-kappaB encoded by a poxvirus.J Virol. 2011 Jan;85(1):264-75. doi: 10.1128/JVI.01149-10. Epub 2010 Oct 27. J Virol. 2011. PMID: 20980501 Free PMC article.
-
Orf Virus ORF120 Protein Positively Regulates the NF-κB Pathway by Interacting with G3BP1.J Virol. 2021 Sep 9;95(19):e0015321. doi: 10.1128/JVI.00153-21. Epub 2021 Sep 9. J Virol. 2021. PMID: 34287041 Free PMC article.
-
A parapoxviral virion protein inhibits NF-κB signaling early in infection.PLoS Pathog. 2017 Aug 7;13(8):e1006561. doi: 10.1371/journal.ppat.1006561. eCollection 2017 Aug. PLoS Pathog. 2017. PMID: 28787456 Free PMC article.
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
Cited by
-
Rescue of a Vaccinia Virus Mutant Lacking IFN Resistance Genes K1L and C7L by the Parapoxvirus Orf Virus.Front Microbiol. 2020 Jul 28;11:1797. doi: 10.3389/fmicb.2020.01797. eCollection 2020. Front Microbiol. 2020. PMID: 32903701 Free PMC article.
-
Poxviral ankyrin proteins.Viruses. 2015 Feb 16;7(2):709-38. doi: 10.3390/v7020709. Viruses. 2015. PMID: 25690795 Free PMC article. Review.
-
Generation of recombinant Orf virus using an enhanced green fluorescent protein reporter gene as a selectable marker.BMC Vet Res. 2011 Dec 22;7:80. doi: 10.1186/1746-6148-7-80. BMC Vet Res. 2011. PMID: 22192523 Free PMC article.
-
An Orf-Virus (ORFV)-Based Vector Expressing a Consensus H1 Hemagglutinin Provides Protection against Diverse Swine Influenza Viruses.Viruses. 2023 Apr 18;15(4):994. doi: 10.3390/v15040994. Viruses. 2023. PMID: 37112974 Free PMC article.
-
Modulation of NF-κB signalling by microbial pathogens.Nat Rev Microbiol. 2011 Apr;9(4):291-306. doi: 10.1038/nrmicro2539. Epub 2011 Mar 8. Nat Rev Microbiol. 2011. PMID: 21383764 Free PMC article. Review.
References
-
- Assarsson, E., J. A. Greenbaum, M. Sundstrom, L. Schaffer, J. A. Hammond, V. Pasquetto, C. Oseroff, R. C. Hendrickson, E. J. Lefkowitz, D. C. Tscharke, J. Sidney, H. M. Grey, S. R. Head, B. Peters, and A. Sette. 2008. Kinetic analysis of a complete poxvirus transcriptome reveals an immediate-early class of genes. Proc. Natl. Acad. Sci. U. S. A. 105:2140-2145. - PMC - PubMed
-
- Bartlett, N., J. A. Symons, D. C. Tscharke, and G. L. Smith. 2002. The vaccinia virus N1L protein is an intracellular homodimer that promotes virulence. J. Gen. Virol. 83:1965-1976. - PubMed
-
- Buss, H., A. Dorrie, M. L. Schmitz, E. Hoffmann, K. Resch, and M. Kracht. 2004. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-κB at serine 536 is mediated by multiple protein kinases including IκB kinase (IKK)-alpha, IKKbeta, IKKepsilon, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J. Biol. Chem. 279:55633-55643. - PubMed
-
- Buttner, M. 1993. Principles of paramunization. Option and limits in veterinary medicine. Comp. Immunol. Microbiol. Infect. Dis. 16:1-10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

