Leishmania actin binds and nicks kDNA as well as inhibits decatenation activity of type II topoisomerase
- PMID: 20147461
- PMCID: PMC2879525
- DOI: 10.1093/nar/gkq051
Leishmania actin binds and nicks kDNA as well as inhibits decatenation activity of type II topoisomerase
Abstract
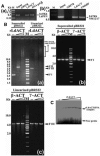
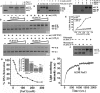
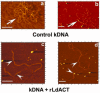
Leishmania actin (LdACT) is an unconventional form of eukaryotic actin in that it markedly differs from other actins in terms of its filament forming as well as toxin and DNase-1-binding properties. Besides being present in the cytoplasm, cortical regions, flagellum and nucleus, it is also present in the kinetoplast where it appears to associate with the kinetoplast DNA (kDNA). However, nothing is known about its role in this organelle. Here, we show that LdACT is indeed associated with the kDNA disc in Leishmania kinetoplast, and under in vitro conditions, it specifically binds DNA primarily through electrostatic interactions involving its unique DNase-1-binding region and the DNA major groove. We further reveal that this protein exhibits DNA-nicking activity which requires its polymeric state as well as ATP hydrolysis and through this activity it converts catenated kDNA minicircles into open form. In addition, we show that LdACT specifically binds bacterial type II topoisomerase and inhibits its decatenation activity. Together, these results strongly indicate that LdACT could play a critical role in kDNA remodeling.
Figures



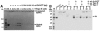
References
-
- Hofmann WA. Cell and molecular biology of nuclear actin. Int. Rev. Cell Mol. Biol. 2009;273:219–263. - PubMed
-
- Louvet E, Percipalle P. Transcriptional control of gene expression by actin and myosin. Int. Rev. Cell Mol. Biol. 2009;272:107–147. - PubMed
-
- Farrants AK. Chromatin remodelling and actin organisation. FEBS Lett. 2008;582:2041–2050. - PubMed
-
- Liu B, Liu Y, Motyka SA, Agbo EE, Englund PT. Fellowship of the rings: the replication of kinetoplast DNA. Trends Parasitol. 2005;21:363–369. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

