Interaction between facilitation and depression at a large CNS synapse reveals mechanisms of short-term plasticity
- PMID: 20147529
- PMCID: PMC6634054
- DOI: 10.1523/JNEUROSCI.4378-09.2010
Interaction between facilitation and depression at a large CNS synapse reveals mechanisms of short-term plasticity
Abstract
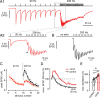
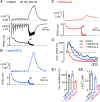
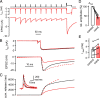
The two fundamental forms of short-term plasticity, short-term depression and facilitation, coexist at most synapses, but little is known about their interaction. Here, we studied the interplay between short-term depression and facilitation at calyx of Held synapses. Stimulation at a "low" frequency of 10 or 20 Hz, which is in the range of the spontaneous activity of these auditory neurons in vivo, induced synaptic depression. Surprisingly, an instantaneous increase of the stimulation frequency to 100 or 200 Hz following the low-frequency train uncovered a robust facilitation of EPSCs relative to the predepressed amplitude level. This facilitation decayed rapidly ( approximately 30 ms) and depended on presynaptic residual Ca(2+), but it was not caused by Ca(2+) current facilitation. To probe the release probability of the remaining readily releasable vesicles following the low-frequency train we made presynaptic Ca(2+) uncaging experiments in the predepressed state of the synapse. We found that low-frequency stimulation depletes the fast-releasable vesicle pool (FRP) down to approximately 40% of control and that the remaining FRP vesicles are released with approximately 2-fold slower release kinetics, indicating a hitherto unknown intrinsic heterogeneity among FRP vesicles. Thus, vesicles with an intrinsically lower release probability predominate after low frequency stimulation and undergo facilitation during the onset of subsequent high-frequency trains. Facilitation in the predepressed state of the synapse might help to stabilize the amount of transmitter release at the onset of high-frequency firing at these auditory synapses.
Figures



Similar articles
-
The reduced release probability of releasable vesicles during recovery from short-term synaptic depression.Neuron. 1999 Aug;23(4):821-32. doi: 10.1016/s0896-6273(01)80039-8. Neuron. 1999. PMID: 10482247
-
Developmental regulation of the intracellular Ca2+ sensitivity of vesicle fusion and Ca2+-secretion coupling at the rat calyx of Held.J Physiol. 2009 Jun 15;587(Pt 12):3009-23. doi: 10.1113/jphysiol.2009.172387. Epub 2009 Apr 29. J Physiol. 2009. PMID: 19403608 Free PMC article.
-
Mechanisms of target-cell specific short-term plasticity at Schaffer collateral synapses onto interneurones versus pyramidal cells in juvenile rats.J Physiol. 2005 Nov 1;568(Pt 3):815-40. doi: 10.1113/jphysiol.2005.093948. Epub 2005 Aug 18. J Physiol. 2005. PMID: 16109728 Free PMC article.
-
The calyx of Held synapse: from model synapse to auditory relay.Annu Rev Physiol. 2012;74:199-224. doi: 10.1146/annurev-physiol-020911-153236. Epub 2011 Oct 24. Annu Rev Physiol. 2012. PMID: 22035348 Review.
-
Vesicle pools and short-term synaptic depression: lessons from a large synapse.Trends Neurosci. 2002 Apr;25(4):206-12. doi: 10.1016/s0166-2236(02)02139-2. Trends Neurosci. 2002. PMID: 11998689 Review.
Cited by
-
Molecular mechanisms that stabilize short term synaptic plasticity during presynaptic homeostatic plasticity.Elife. 2018 Nov 13;7:e40385. doi: 10.7554/eLife.40385. Elife. 2018. PMID: 30422113 Free PMC article.
-
The number of components of enhancement contributing to short-term synaptic plasticity at the neuromuscular synapse during patterned nerve Stimulation progressively decreases as basal release probability is increased from low to normal levels by changing extracellular Ca2+.J Neurosci. 2011 May 11;31(19):7060-72. doi: 10.1523/JNEUROSCI.0392-11.2011. J Neurosci. 2011. PMID: 21562268 Free PMC article.
-
Functional Postnatal Maturation of the Medial Olivocochlear Efferent-Outer Hair Cell Synapse.J Neurosci. 2020 Jun 17;40(25):4842-4857. doi: 10.1523/JNEUROSCI.2409-19.2020. Epub 2020 May 19. J Neurosci. 2020. PMID: 32430293 Free PMC article.
-
Different states of synaptic vesicle priming explain target cell type-dependent differences in neurotransmitter release.Proc Natl Acad Sci U S A. 2024 Apr 30;121(18):e2322550121. doi: 10.1073/pnas.2322550121. Epub 2024 Apr 24. Proc Natl Acad Sci U S A. 2024. PMID: 38657053 Free PMC article.
-
A sequential two-step priming scheme reproduces diversity in synaptic strength and short-term plasticity.Proc Natl Acad Sci U S A. 2022 Aug 23;119(34):e2207987119. doi: 10.1073/pnas.2207987119. Epub 2022 Aug 15. Proc Natl Acad Sci U S A. 2022. PMID: 35969787 Free PMC article.
References
-
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron. 2003;38:79–88. - PubMed
-
- Boudkkazi S, Carlier E, Ankri N, Caillard O, Giraud P, Fronzaroli-Molinieres L, Debanne D. Release-dependent variations in synaptic latency: a putative code for short- and long-term synaptic dynamics. Neuron. 2007;56:1048–1060. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
