Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice
- PMID: 20147530
- PMCID: PMC6634037
- DOI: 10.1523/JNEUROSCI.5693-09.2010
Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice
Abstract
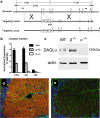
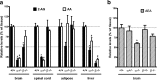
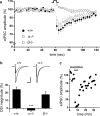
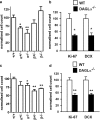
Endocannabinoids (eCBs) function as retrograde signaling molecules at synapses throughout the brain, regulate axonal growth and guidance during development, and drive adult neurogenesis. There remains a lack of genetic evidence as to the identity of the enzyme(s) responsible for the synthesis of eCBs in the brain. Diacylglycerol lipase-alpha (DAGLalpha) and -beta (DAGLbeta) synthesize 2-arachidonoyl-glycerol (2-AG), the most abundant eCB in the brain. However, their respective contribution to this and to eCB signaling has not been tested. In the present study, we show approximately 80% reductions in 2-AG levels in the brain and spinal cord in DAGLalpha(-/-) mice and a 50% reduction in the brain in DAGLbeta(-/-) mice. In contrast, DAGLbeta plays a more important role than DAGLalpha in regulating 2-AG levels in the liver, with a 90% reduction seen in DAGLbeta(-/-) mice. Levels of arachidonic acid decrease in parallel with 2-AG, suggesting that DAGL activity controls the steady-state levels of both lipids. In the hippocampus, the postsynaptic release of an eCB results in the transient suppression of GABA-mediated transmission at inhibitory synapses; we now show that this form of synaptic plasticity is completely lost in DAGLalpha(-/-) animals and relatively unaffected in DAGLbeta(-/-) animals. Finally, we show that the control of adult neurogenesis in the hippocampus and subventricular zone is compromised in the DAGLalpha(-/-) and/or DAGLbeta(-/-) mice. These findings provide the first evidence that DAGLalpha is the major biosynthetic enzyme for 2-AG in the nervous system and reveal an essential role for this enzyme in regulating retrograde synaptic plasticity and adult neurogenesis.
Figures

References
-
- Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, Marsicano G, Kokaia Z, Guzmán M, Galve-Roperh I. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704–1706. - PubMed
-
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urbán GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. - PubMed
-
- Bilsland JG, Haldon C, Goddard J, Oliver K, Murray F, Wheeldon A, Cumberbatch J, McAllister G, Munoz-Sanjuan I. A rapid method for the quantification of mouse hippocampal neurogenesis in vivo by flow cytometry. Validation with conventional and enhanced immunohistochemical methods. J Neurosci Methods. 2006;157:54–63. - PubMed
-
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
