CSF biomarkers in prediction of cerebral and clinical change in mild cognitive impairment and Alzheimer's disease
- PMID: 20147537
- PMCID: PMC2828879
- DOI: 10.1523/JNEUROSCI.3785-09.2010
CSF biomarkers in prediction of cerebral and clinical change in mild cognitive impairment and Alzheimer's disease
Abstract
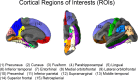
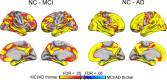
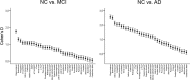
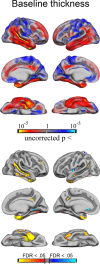
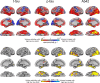
Brain atrophy and altered CSF levels of amyloid beta (Abeta(42)) and the microtubule-associated protein tau are potent biomarkers of Alzheimer's disease (AD)-related pathology. However, the relationship between CSF biomarkers and brain morphometry is poorly understood. Thus, we addressed the following questions. (1) Can CSF biomarker levels explain the morphometric differences between normal controls (NC) and patients with mild cognitive impairment (MCI) or AD? (2) How are CSF biomarkers related to atrophy across the brain? (3) How closely are CSF biomarkers and morphometry related to clinical change [clinical dementia rating sum of boxes (CDR-sb)]? Three hundred seventy participants (105 NC, 175 MCI, 90 AD) from the Alzheimer's Disease Neuroimaging Initiative were studied, of whom 309 were followed for 1 year and 176 for 2 years. Analyses were performed across the entire cortical surface, as well as for 30 cortical and subcortical regions of interest. Results showed that CSF biomarker levels could not account for group differences in brain morphometry at baseline but that CSF biomarker levels showed moderate relationships to longitudinal atrophy rates in numerous brain areas, not restricted to medial temporal structures. Baseline morphometry was at least as predictive of atrophy as were CSF biomarkers. Even MCI patients with levels of Abeta(42) comparable with controls and of p-tau lower than controls showed more atrophy than the controls. Morphometry predicted change in CDR-sb better than did CSF biomarkers. These results indicate that morphometric changes in MCI and AD are not secondary to CSF biomarker changes and that the two types of biomarkers yield complementary information.
Figures


References
-
- Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology. 1992;42:1681–1688. - PubMed
-
- Braak H, Braak E. On areas of transition between entorhinal allocortex and temporal isocortex in the human brain. Normal morphology and lamina-specific pathology in Alzheimer's disease. Acta Neuropathol. 1985;68:325–332. - PubMed
-
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. - PubMed
-
- Chou YY, Leporé N, Avedissian C, Madsen SK, Parikshak N, Hua X, Shaw LM, Trojanowski JQ, Weiner MW, Toga AW, Thompson PM. Mapping correlations between ventricular expansion and CSF amyloid and tau biomarkers in 240 subjects with Alzheimer's disease, mild cognitive impairment and elderly controls. Neuroimage. 2009;46:394–410. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
