Reevaluation of neurodegeneration in lurcher mice: constitutive ion fluxes cause cell death with, not by, autophagy
- PMID: 20147545
- PMCID: PMC6634053
- DOI: 10.1523/JNEUROSCI.6030-09.2010
Reevaluation of neurodegeneration in lurcher mice: constitutive ion fluxes cause cell death with, not by, autophagy
Abstract
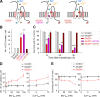
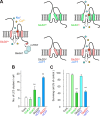
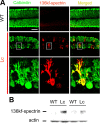
The lurcher (Lc) mice have served as a valuable model for neurodegeneration for decades. Although the responsible mutation was identified in genes encoding delta2 glutamate receptors (GluD2s), which are predominantly expressed in cerebellar Purkinje cells, how the mutant receptor (GluD2(Lc)) triggers cell death has remained elusive. Here, taking advantage of recent knowledge about the domain structure of GluD2, we reinvestigated Lc-mediated cell death, focusing on the "autophagic cell death" hypothesis. Although autophagy and cell death were induced by the expression of GluD2(Lc) in heterologous cells and cultured neurons, they were blocked by the introduction of mutations in the channel pore domain of GluD2(Lc) or by removal of extracellular Na(+). In addition, although GluD2(Lc) is reported to directly activate autophagy, mutant channels that are not associated with n-PIST (neuronal isoform of protein-interacting specifically with TC10)-Beclin1 still caused autophagy and cell death. Furthermore, cells expressing GluD2(Lc) showed decreased ATP levels and increased AMP-activated protein kinase (AMPK) activities in a manner dependent on extracellular Na(+). Thus, constitutive currents were likely necessary and sufficient to induce autophagy via AMPK activation, regardless of the n-PIST-Beclin1 pathway in vitro. Interestingly, the expression of dominant-negative AMPK suppressed GluD2(Lc)-induced autophagy but did not prevent cell death in heterologous cells. Similarly, the disruption of Atg5, a gene crucial for autophagy, did not prevent but rather aggravated Purkinje-cell death in Lc mice. Furthermore, calpains were specifically activated in Lc Purkinje cells. Together, these results suggest that Lc-mediated cell death was not caused by autophagy but necrosis with autophagic features both in vivo and in vitro.
Figures





Similar articles
-
A novel protein complex linking the delta 2 glutamate receptor and autophagy: implications for neurodegeneration in lurcher mice.Neuron. 2002 Aug 29;35(5):921-33. doi: 10.1016/s0896-6273(02)00861-9. Neuron. 2002. PMID: 12372286
-
Excitotoxicity and autophagy: lurcher may not be a model of "autophagic cell death".Autophagy. 2010 May;6(4):568-70. doi: 10.4161/auto.6.4.11951. Epub 2010 May 16. Autophagy. 2010. PMID: 20404558
-
Lurcher GRID2-induced death and depolarization can be dissociated in cerebellar Purkinje cells.Neuron. 2003 Mar 6;37(5):813-9. doi: 10.1016/s0896-6273(03)00093-x. Neuron. 2003. PMID: 12628171
-
Mechanisms of compartmental purkinje cell death and survival in the lurcher mutant mouse.Cerebellum. 2011 Sep;10(3):504-14. doi: 10.1007/s12311-010-0231-4. Cerebellum. 2011. PMID: 21104177 Review.
-
The ins and outs of GluD2--why and how Purkinje cells use the special glutamate receptor.Cerebellum. 2012 Jun;11(2):438-9. doi: 10.1007/s12311-011-0328-4. Cerebellum. 2012. PMID: 22113500 Review.
Cited by
-
IP3R1 deficiency in the cerebellum/brainstem causes basal ganglia-independent dystonia by triggering tonic Purkinje cell firings in mice.Front Neural Circuits. 2013 Oct 4;7:156. doi: 10.3389/fncir.2013.00156. eCollection 2013. Front Neural Circuits. 2013. PMID: 24109434 Free PMC article.
-
Innate immunity in the Grid2Lc/+ mouse model of cerebellar neurodegeneration: glial CD95/CD95L plays a non-apoptotic role in persistent neuron loss-associated inflammatory reactions in the cerebellum.J Neuroinflammation. 2013 May 15;10:65. doi: 10.1186/1742-2094-10-65. J Neuroinflammation. 2013. PMID: 23672668 Free PMC article.
-
Autophagy: a potential key contributor to the therapeutic action of mesenchymal stem cells.Autophagy. 2020 Jan;16(1):28-37. doi: 10.1080/15548627.2019.1630223. Epub 2019 Jun 18. Autophagy. 2020. PMID: 31185790 Free PMC article. Review.
-
Caspase-3 Mediated Cell Death in the Normal Development of the Mammalian Cerebellum.Int J Mol Sci. 2018 Dec 12;19(12):3999. doi: 10.3390/ijms19123999. Int J Mol Sci. 2018. PMID: 30545052 Free PMC article. Review.
-
Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer's-like axonal dystrophy.J Neurosci. 2011 May 25;31(21):7817-30. doi: 10.1523/JNEUROSCI.6412-10.2011. J Neurosci. 2011. PMID: 21613495 Free PMC article.
References
-
- Ames A., 3rd CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34:42–68. - PubMed
-
- Bangalore R, Mehrke G, Gingrich K, Hofmann F, Kass RS. Influence of L-type Ca channel alpha 2/delta-subunit on ionic and gating current in transiently transfected HEK 293 cells. Am J Physiol. 1996;270:H1521–H1528. - PubMed
-
- Barros LF, Hermosilla T, Castro J. Necrotic volume increase and the early physiology of necrosis. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:401–409. - PubMed
-
- Borsello T, Croquelois K, Hornung JP, Clarke PG. N-methyl-d-aspartate-triggered neuronal death in organotypic hippocampal cultures is endocytic, autophagic and mediated by the c-Jun N-terminal kinase pathway. Eur J Neurosci. 2003;18:473–485. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
