VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety
- PMID: 20147547
- PMCID: PMC6634029
- DOI: 10.1523/JNEUROSCI.5196-09.2010
VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety
Abstract
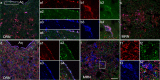
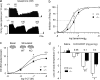
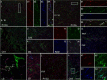
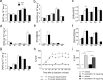
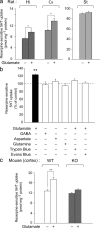
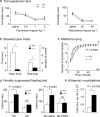
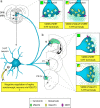
Three different subtypes of H(+)-dependent carriers (named VGLUT1-3) concentrate glutamate into synaptic vesicles before its exocytotic release. Neurons using other neurotransmitter than glutamate (such as cholinergic striatal interneurons and 5-HT neurons) express VGLUT3. It was recently reported that VGLUT3 increases acetylcholine vesicular filling, thereby, stimulating cholinergic transmission. This new regulatory mechanism is herein designated as vesicular-filling synergy (or vesicular synergy). In the present report, we found that deletion of VGLUT3 increased several anxiety-related behaviors in adult and in newborn mice as early as 8 d after birth. This precocious involvement of a vesicular glutamate transporter in anxiety led us to examine the underlying functional implications of VGLUT3 in 5-HT neurons. On one hand, VGLUT3 deletion caused a significant decrease of 5-HT(1A)-mediated neurotransmission in raphe nuclei. On the other hand, VGLUT3 positively modulated 5-HT transmission of a specific subset of 5-HT terminals from the hippocampus and the cerebral cortex. VGLUT3- and VMAT2-positive serotonergic fibers show little or no 5-HT reuptake transporter. These results unravel the existence of a novel subset of 5-HT terminals in limbic areas that might play a crucial role in anxiety-like behaviors. In summary, VGLUT3 accelerates 5-HT transmission at the level of specific 5-HT terminals and can exert an inhibitory control at the raphe level. Furthermore, our results suggest that the loss of VGLUT3 expression leads to anxiety-associated behaviors and should be considered as a potential new target for the treatment of this disorder.
Figures

References
-
- Boulland JL, Qureshi T, Seal RP, Rafiki A, Gundersen V, Bergersen LH, Fremeau RT, Jr, Edwards RH, Storm-Mathisen J, Chaudhry FA. Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. J Comp Neurol. 2004;480:264–280. - PubMed
-
- Branchi I, Santucci D, Alleva E. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res. 2001;125:49–56. - PubMed
-
- Caliendo G, Santagada V, Perissutti E, Fiorino F. Derivatives as 5HT1A receptor ligands–past and present. Curr Med Chem. 2005;12:1721–1753. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
