Ecto-5'-nucleotidase (CD73) inhibits nociception by hydrolyzing AMP to adenosine in nociceptive circuits
- PMID: 20147550
- PMCID: PMC2826808
- DOI: 10.1523/JNEUROSCI.5324-09.2010
Ecto-5'-nucleotidase (CD73) inhibits nociception by hydrolyzing AMP to adenosine in nociceptive circuits
Abstract
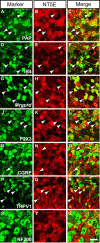
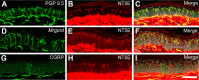
Ecto-5'-nucleotidase (NT5E, CD73) is a membrane-anchored protein that hydrolyzes extracellular adenosine 5'-monophosphate (AMP) to adenosine in diverse tissues but has not been directly studied in nociceptive neurons. We found that NT5E was located on peptidergic and nonpeptidergic nociceptive neurons in dorsal root ganglia (DRG) and on axon terminals in lamina II (the substantia gelatinosa) of spinal cord. NT5E was also located on epidermal keratinocytes, cells of the dermis, and on nociceptive axon terminals in the epidermis. Following nerve injury, NT5E protein and AMP histochemical staining were coordinately reduced in lamina II. In addition, AMP hydrolytic activity was reduced in DRG neurons and spinal cord of Nt5e(-/-) mice. The antinociceptive effects of AMP, when combined with the adenosine kinase inhibitor 5-iodotubericidin, were reduced by approximately 50% in Nt5e(-/-) mice and were eliminated in Adenosine A(1) receptor (A(1)R, Adora1) knock-out mice. Additionally, Nt5e(-/-) mice displayed enhanced sensitivity in the tail immersion assay, in the complete Freund's adjuvant model of inflammatory pain and in the spared nerve injury model of neuropathic pain. Collectively, our data indicate that the ectonucleotidase NT5E regulates nociception by hydrolyzing AMP to adenosine in nociceptive circuits and represents a new molecular target for the treatment of chronic pain. Moreover, our data suggest NT5E is well localized to regulate nucleotide signaling between skin cells and sensory axons.
Figures





Similar articles
-
PAP and NT5E inhibit nociceptive neurotransmission by rapidly hydrolyzing nucleotides to adenosine.Mol Pain. 2011 Oct 19;7:80. doi: 10.1186/1744-8069-7-80. Mol Pain. 2011. PMID: 22011440 Free PMC article.
-
Recombinant ecto-5'-nucleotidase (CD73) has long lasting antinociceptive effects that are dependent on adenosine A1 receptor activation.Mol Pain. 2010 Apr 14;6:20. doi: 10.1186/1744-8069-6-20. Mol Pain. 2010. PMID: 20398264 Free PMC article.
-
Tissue-nonspecific alkaline phosphatase acts redundantly with PAP and NT5E to generate adenosine in the dorsal spinal cord.J Neurosci. 2013 Jul 3;33(27):11314-22. doi: 10.1523/JNEUROSCI.0133-13.2013. J Neurosci. 2013. PMID: 23825434 Free PMC article.
-
Cell type- and tissue-specific functions of ecto-5'-nucleotidase (CD73).Am J Physiol Cell Physiol. 2019 Dec 1;317(6):C1079-C1092. doi: 10.1152/ajpcell.00285.2019. Epub 2019 Aug 28. Am J Physiol Cell Physiol. 2019. PMID: 31461341 Free PMC article. Review.
-
Controlling the Immune Suppressor: Transcription Factors and MicroRNAs Regulating CD73/NT5E.Front Immunol. 2018 Apr 18;9:813. doi: 10.3389/fimmu.2018.00813. eCollection 2018. Front Immunol. 2018. PMID: 29720980 Free PMC article. Review.
Cited by
-
FACS array profiling identifies Ecto-5' nucleotidase as a striatopallidal neuron-specific gene involved in striatal-dependent learning.J Neurosci. 2013 May 15;33(20):8794-809. doi: 10.1523/JNEUROSCI.2989-12.2013. J Neurosci. 2013. PMID: 23678122 Free PMC article.
-
Temporal Single Cell Analysis of Leukemia Microenvironment Identifies Taurine-Taurine Transporter Axis as a Key Regulator of Myeloid Leukemia.bioRxiv [Preprint]. 2024 May 14:2024.05.11.593633. doi: 10.1101/2024.05.11.593633. bioRxiv. 2024. PMID: 38798540 Free PMC article. Preprint.
-
Pain-relieving prospects for adenosine receptors and ectonucleotidases.Trends Mol Med. 2011 Apr;17(4):188-96. doi: 10.1016/j.molmed.2010.12.006. Epub 2011 Jan 13. Trends Mol Med. 2011. PMID: 21236731 Free PMC article. Review.
-
Distribution of ecto-nucleotidases in mouse sensory circuits suggests roles for nucleoside triphosphate diphosphohydrolase-3 in nociception and mechanoreception.Neuroscience. 2011 Oct 13;193:387-98. doi: 10.1016/j.neuroscience.2011.07.044. Epub 2011 Jul 27. Neuroscience. 2011. PMID: 21807070 Free PMC article.
-
Mechanisms of ATP release in pain: role of pannexin and connexin channels.Purinergic Signal. 2021 Dec;17(4):549-561. doi: 10.1007/s11302-021-09822-6. Epub 2021 Nov 18. Purinergic Signal. 2021. PMID: 34792743 Free PMC article. Review.
References
-
- Bantel C, Tobin JR, Li X, Childers SR, Chen SR, Eisenach JC. Intrathecal adenosine following spinal nerve ligation in rat: short residence time in cerebrospinal fluid and no change in A(1) receptor binding. Anesthesiology. 2002;96:103–108. - PubMed
-
- Belfrage M, Segerdahl M, Arnér S, Sollevi A. The safety and efficacy of intrathecal adenosine in patients with chronic neuropathic pain. Anesth Analg. 1999;89:136–142. - PubMed
-
- Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett. 1996;206:33–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
