Multisegmental A{delta}- and C-fiber input to neurons in lamina I and the lateral spinal nucleus
- PMID: 20147564
- PMCID: PMC6634039
- DOI: 10.1523/JNEUROSCI.3445-09.2010
Multisegmental A{delta}- and C-fiber input to neurons in lamina I and the lateral spinal nucleus
Abstract
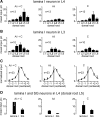
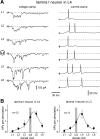
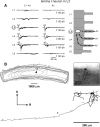
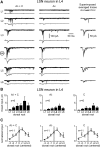
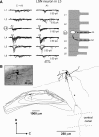
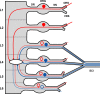
Spinal lamina I and the lateral spinal nucleus (LSN) receive and integrate nociceptive primary afferent inputs to project through diverse ascending pathways. The pattern of the afferent supply of individual lamina I and LSN neurons through different segmental dorsal roots is poorly understood. Therefore, we recorded responses of lamina I and LSN neurons in spinal segments L4 and L3 to stimulation of six ipsilateral dorsal roots (L1-L6). The neurons were viewed through the overlying white matter in the isolated spinal cord preparation using the oblique infrared LED illumination technique. Orientation of myelinated fibers in the white matter was used as a criterion to distinguish between the LSN and lamina I. Both types of neurons received mixed (monosynaptic and polysynaptic) excitatory Adelta- and C-fiber input from up to six dorsal roots, with only less than one-third of it arising from the corresponding segmental root. The largest mixed input arose from the dorsal root of the neighboring caudal segment. Lamina I and LSN neurons could fire spikes upon the stimulation of up to six different dorsal roots. We also found that individual lamina I neurons can receive converging monosynaptic Adelta- and/or C-fiber inputs from up to six segmental roots. This study shows that lamina I and LSN neurons function as intersegmental integrators of primary afferent inputs. We suggest that broad monosynaptic convergence of Adelta- and C-afferents onto a lamina I neuron is important for the somatosensory processing.
Figures




Similar articles
-
Monosynaptic convergence of C- and Adelta-afferent fibres from different segmental dorsal roots on to single substantia gelatinosa neurones in the rat spinal cord.J Physiol. 2008 Sep 1;586(17):4165-77. doi: 10.1113/jphysiol.2008.154898. Epub 2008 Jul 17. J Physiol. 2008. PMID: 18635648 Free PMC article.
-
Diverse firing properties and Aβ-, Aδ-, and C-afferent inputs of small local circuit neurons in spinal lamina I.Pain. 2016 Feb;157(2):475-487. doi: 10.1097/j.pain.0000000000000394. Pain. 2016. PMID: 26797505
-
Contralateral Afferent Input to Lumbar Lamina I Neurons as a Neural Substrate for Mirror-Image Pain.J Neurosci. 2023 May 3;43(18):3245-3258. doi: 10.1523/JNEUROSCI.1897-22.2023. Epub 2023 Mar 22. J Neurosci. 2023. PMID: 36948583 Free PMC article.
-
Low- and high-threshold primary afferent inputs to spinal lamina III antenna-type neurons.Pain. 2018 Nov;159(11):2214-2222. doi: 10.1097/j.pain.0000000000001320. Pain. 2018. PMID: 29939963
-
Action in primary afferent fibers in the spinal cord.Int J Neurosci. 1970 Oct;1(1):1-25. doi: 10.3109/00207457009147614. Int J Neurosci. 1970. PMID: 4349423 Review. No abstract available.
Cited by
-
Spinal cord involvement in Lewy body-related α-synucleinopathies.J Spinal Cord Med. 2020 Nov;43(6):832-845. doi: 10.1080/10790268.2018.1557863. Epub 2019 Jan 8. J Spinal Cord Med. 2020. PMID: 30620687 Free PMC article.
-
Surround Inhibition Mediates Pain Relief by Low Amplitude Spinal Cord Stimulation: Modeling and Measurement.eNeuro. 2022 Oct 5;9(5):ENEURO.0058-22.2022. doi: 10.1523/ENEURO.0058-22.2022. Print 2022 Sep-Oct. eNeuro. 2022. PMID: 36150892 Free PMC article.
-
Non-peptidergic small diameter primary afferents expressing VGluT2 project to lamina I of mouse spinal dorsal horn.Mol Pain. 2011 Dec 8;7:95. doi: 10.1186/1744-8069-7-95. Mol Pain. 2011. PMID: 22152428 Free PMC article.
-
Reliability of task-based fMRI in the dorsal horn of the human spinal cord.bioRxiv [Preprint]. 2024 Jun 25:2023.12.22.572825. doi: 10.1101/2023.12.22.572825. bioRxiv. 2024. Update in: Imaging Neurosci (Camb). 2024 Aug 22;2:imag-2-00273. doi: 10.1162/imag_a_00273. PMID: 38187724 Free PMC article. Updated. Preprint.
-
Involvement of propriospinal processes in conditioned pain modulation.Pain. 2024 Sep 1;165(9):1907-1913. doi: 10.1097/j.pain.0000000000003217. Epub 2024 Mar 27. Pain. 2024. PMID: 38537057 Review. No abstract available.
References
-
- Amaral DG. The functional organization of perception and movement. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science. Ed 4. New York: McGraw-Hill; 2000. pp. 337–348.
-
- Burstein R, Cliffer KD, Giesler GJ., Jr Cells of origin of the spinohypothalamic tract in the rat. J Comp Neurol. 1990a;291:329–344. - PubMed
-
- Burstein R, Dado RJ, Giesler GJ., Jr The cells of origin of the spinothalamic tract of the rat: a quantitative reexamination. Brain Res. 1990b;511:329–337. - PubMed
-
- Cervero F, Connell LA. Distribution of somatic and visceral primary afferent fibers within the thoracic spinal cord of the cat. J Comp Neurol. 1984a;230:88–98. - PubMed
-
- Cervero F, Connell LA. Fine afferent fibers from viscera do not terminate in the substantia gelatinosa of the thoracic spinal cord. Brain Res. 1984b;294:370–374. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
