Electrostatics of aquaporin and aquaglyceroporin channels correlates with their transport selectivity
- PMID: 20147624
- PMCID: PMC2819975
- DOI: 10.1073/pnas.0910632107
Electrostatics of aquaporin and aquaglyceroporin channels correlates with their transport selectivity
Abstract
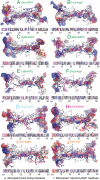
Aquaporins are homotetrameric channel proteins, which allow the diffusion of water and small solutes across biological membranes. According to their transport function, aquaporins can be divided into "orthodox aquaporins", which allow the flux of water molecules only, and "aquaglyceroporins", which facilitate the diffusion of glycerol and other small solutes in addition to water. The contribution of individual residues in the pore to the selectivity of orthodox aquaporins and aquaglyceroporins is not yet fully understood. To gain insights into aquaporin selectivity, we focused on the sequence variation and electrostatics of their channels. The continuum Poisson-Boltzmann electrostatic potential along the channel was calculated and compared for ten three-dimensional-structures which are representatives of different aquaporin subfamilies, and a panel of functionally characterized mutants, for which high-accuracy three-dimensional-models could be derived. Interestingly, specific electrostatic profiles associated with the main selectivity to water or glycerol could be identified. In particular: (i) orthodox aquaporins showed a distinctive electrostatic potential maximum at the periplasmic side of the channel around the aromatic/Arg (ar/R) constriction site; (ii) aquaporin-0 (AQP0), a mammalian aquaporin with considerably low water permeability, had an additional deep minimum at the cytoplasmic side; (iii) aquaglyceroporins showed a rather flat potential all along the channel; and (iv) the bifunctional protozoan PfAQP had an unusual all negative profile. Evaluation of electrostatics of the mutants, along with a thorough sequence analysis of the aquaporin pore-lining residues, illuminated the contribution of specific residues to the electrostatics of the channels and possibly to their selectivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

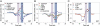
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

