Neisseria gonorrhoeae enhances HIV-1 infection of primary resting CD4+ T cells through TLR2 activation
- PMID: 20147631
- PMCID: PMC3739425
- DOI: 10.4049/jimmunol.0902125
Neisseria gonorrhoeae enhances HIV-1 infection of primary resting CD4+ T cells through TLR2 activation
Abstract
Sexually transmitted infections increase the likelihood of HIV-1 transmission. We investigated the effect of Neisseria gonorrheae (gonococcus [GC]) exposure on HIV replication in primary resting CD4(+) T cells, a major HIV target cell during the early stage of sexual transmission of HIV. GC and TLR2 agonists, such as peptidylglycan (PGN), Pam(3)CSK(4), and Pam(3)C-Lip, a GC-derived synthetic lipopeptide, but not TLR4 agonists including LPS or GC lipooligosaccharide enhanced HIV-1 infection of primary resting CD4(+) T cells after viral entry. Pretreatment of CD4(+) cells with PGN also promoted HIV infection. Anti-TLR2 Abs abolished the HIV enhancing effect of GC and Pam(3)C-Lip, indicating that GC-mediated enhancement of HIV infection of resting CD4(+) T cells was through TLR2. IL-2 was required for TLR2-mediated HIV enhancement. PGN and GC induced cell surface expression of T cell activation markers and HIV coreceptors, CCR5 and CXCR4. The maximal postentry HIV enhancing effect was achieved when PGN was added immediately after viral exposure. Kinetic studies and analysis of HIV DNA products indicated that GC exposure and TLR2 activation enhanced HIV infection at the step of nuclear import. We conclude that GC enhanced HIV infection of primary resting CD4(+) T cells through TLR2 activation, which both increased the susceptibility of primary CD4(+) T cells to HIV infection as well as enhanced HIV-infected CD4(+) T cells at the early stage of HIV life cycle after entry. This study provides a molecular mechanism by which nonulcerative sexually transmitted infections mediate enhancement of HIV infection and has implication for HIV prevention and therapeutics.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures

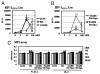




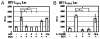
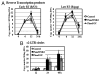
Similar articles
-
TLR2 activation enhances HIV nuclear import and infection through T cell activation-independent and -dependent pathways.J Immunol. 2012 Feb 1;188(3):992-1001. doi: 10.4049/jimmunol.1102098. Epub 2011 Dec 30. J Immunol. 2012. PMID: 22210918 Free PMC article.
-
Toll-Like Receptor 2 Ligation Enhances HIV-1 Replication in Activated CCR6+ CD4+ T Cells by Increasing Virus Entry and Establishing a More Permissive Environment to Infection.J Virol. 2017 Jan 31;91(4):e01402-16. doi: 10.1128/JVI.01402-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27928019 Free PMC article.
-
Neisseria gonorrhoeae-induced human defensins 5 and 6 increase HIV infectivity: role in enhanced transmission.J Immunol. 2008 May 1;180(9):6176-85. doi: 10.4049/jimmunol.180.9.6176. J Immunol. 2008. PMID: 18424739 Free PMC article.
-
Modulation of HIV transmission by Neisseria gonorrhoeae: molecular and immunological aspects.Curr HIV Res. 2012 Apr;10(3):211-7. doi: 10.2174/157016212800618138. Curr HIV Res. 2012. PMID: 22384840 Free PMC article. Review.
-
Dissecting How CD4 T Cells Are Lost During HIV Infection.Cell Host Microbe. 2016 Mar 9;19(3):280-91. doi: 10.1016/j.chom.2016.02.012. Cell Host Microbe. 2016. PMID: 26962940 Free PMC article. Review.
Cited by
-
A virus-packageable CRISPR screen identifies host factors mediating interferon inhibition of HIV.Elife. 2018 Dec 6;7:e39823. doi: 10.7554/eLife.39823. Elife. 2018. PMID: 30520725 Free PMC article.
-
Neisseria gonorrhoeae co-infection exacerbates vaginal HIV shedding without affecting systemic viral loads in human CD34+ engrafted mice.PLoS One. 2018 Jan 23;13(1):e0191672. doi: 10.1371/journal.pone.0191672. eCollection 2018. PLoS One. 2018. PMID: 29360873 Free PMC article.
-
TLR2 activation enhances HIV nuclear import and infection through T cell activation-independent and -dependent pathways.J Immunol. 2012 Feb 1;188(3):992-1001. doi: 10.4049/jimmunol.1102098. Epub 2011 Dec 30. J Immunol. 2012. PMID: 22210918 Free PMC article.
-
Nuclear receptor signaling inhibits HIV-1 replication in macrophages through multiple trans-repression mechanisms.J Virol. 2011 Oct;85(20):10834-50. doi: 10.1128/JVI.00789-11. Epub 2011 Aug 17. J Virol. 2011. PMID: 21849441 Free PMC article.
-
Chlamydia trachomatis Enhances HIV Infection of Non-Activated PBMCs.EC Microbiol. 2022 Apr;18(4):13-17. Epub 2022 Mar 8. EC Microbiol. 2022. PMID: 36507927 Free PMC article.
References
-
- Cohen MS, Miller WC. Sexually transmitted diseases and human immunodeficiency virus infection: cause, effect, or both? Int J Infect Dis. 1998;3:1–4. - PubMed
-
- Plummer FA, Simonsen JN, Cameron DW, Ndinya-Achola JO, Kreiss JK, Gakinya MN, Waiyaki P, Cheang M, Piot P, Ronald AR, et al. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1991;163:233–239. - PubMed
-
- Cameron DW, Simonsen JN, D’Costa LJ, Ronald AR, Maitha GM, Gakinya MN, Cheang M, Ndinya-Achola JO, Piot P, Brunham RC, et al. Female to male transmission of human immunodeficiency virus type 1: risk factors for seroconversion in men. Lancet. 1989;2:403–407. - PubMed
-
- Ghys PD, Fransen K, Diallo MO, Ettiègne-Traoré V, Coulibaly IM, Yeboué KM, Kalish ML, Maurice C, Whitaker JP, Greenberg AE, Laga M. The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Côte d’Ivoire. AIDS. 1997;11:F85–F93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

