Aspergillus PCR: one step closer to standardization
- PMID: 20147637
- PMCID: PMC2849576
- DOI: 10.1128/JCM.01767-09
Aspergillus PCR: one step closer to standardization
Abstract
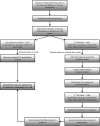
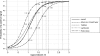
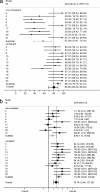
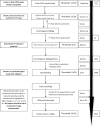
PCR has been used as an aid in the diagnosis of invasive aspergillosis for almost 2 decades. A lack of standardization has limited both its acceptance as a diagnostic tool and multicenter clinical evaluations, preventing its inclusion in disease-defining criteria. In 2006, the European Aspergillus PCR Initiative was formed. The aim of the initiative was to provide optimal standardized protocols for the widespread clinical evaluation of the Aspergillus PCR to determine its diagnostic role and allow inclusion in disease diagnosis criteria. Quality control panels were developed and circulated to centers for evaluation of the existing methodology before recommendations based on the initial results were proposed for further panels. The centers were anonymously classified as "compliant" or "noncompliant," according to whether they had followed the proposed recommendations before the performance parameters were determined and meta-regression analysis was performed. Most PCR amplification systems provided similar detection thresholds, although positivity was a function of the fungal burden. When PCR amplification was combined with DNA extraction, 50% of the centers failed to achieve the same level of detection. Meta-regression analysis showed positive correlations between sensitivity and extraction protocols incorporating the proposed recommendations and the use of bead beating, white cell lysis buffer, and an internal control PCR. The use of elution volumes above 100 microl showed a negative correlation with sensitivity. The efficiency of the Aspergillus PCR is limited by the extraction procedure and not by PCR amplification. For PCR testing of whole blood, it is essential that large blood volumes (>or=3 ml) be efficiently lysed before bead beating to disrupt the fungal cell and performance of an internal control PCR to exclude false negativity. DNA should be eluted in volumes of <100 microl.
Figures
References
-
- Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, T. J. Walsh, and the Invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer, Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. - PubMed
-
- Bossuyt, P. M., J. B. Reitsma, D. E. Bruns, C. A. Gatsonis, P. P. Glasziou, L. M. Irwig, J. G. Lijmer, D. Moher, D. Rennie, H. C. de Vet, and the STARD Group. 2003. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Fam. Pract. 21:4-10. - PubMed
-
- Caillot, D., J. F. Couaillier, A. Bernard, O. Casasnovas, D. W. Denning, L. Mannone, J. Lopez, G. Couillault, F. Piard, O. Vagner, and H. Guy. 2001. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J. Clin. Oncol. 19:253-259. - PubMed
-
- De Pauw, B., T. J. Walsh, J. P. Donnelly, D. A. Stevens, J. E. Edwards, T. Calandra, P. G. Pappas, J. Maertens, O. Lortholary, C. A. Kauffman, D. W. Denning, T. F. Patterson, G. Maschmeyer, J. Bille, W. E. Dismukes, R. Herbrecht, W. W. Hope, C. C. Kibbler, B. J. Kullberg, K. A. Marr, P. Muñoz, F. C. Odds, J. R. Perfect, A. Restrepo, M. Ruhnke, B. H. Segal, J. D. Sobel, T. C. Sorrell, C. Viscoli, J. R. Wingard, T. Zaoutis, J. E. Bennett, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group, and National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813-1821. - PMC - PubMed
-
- Donnelly, J. P. 2006. Polymerase chain reaction for diagnosing invasive aspergillosis: getting closer but still a ways to go. Clin. Infect. Dis. 42:487-489. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

