Multicenter external quality assessment of molecular methods for detection of human herpesvirus 6
- PMID: 20147642
- PMCID: PMC2897485
- DOI: 10.1128/JCM.01145-09
Multicenter external quality assessment of molecular methods for detection of human herpesvirus 6
Abstract
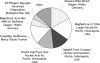
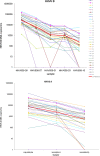
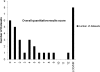
The purpose of this study was to evaluate the performance of laboratories for the detection and quantification of human herpesvirus 6 (HHV-6) by an external quality assessment (EQA) evaluation. The HHV-6 EQA panel consisted of eight samples containing various concentrations of HHV-6 type A (strain GS) or type B (strain Z29), two samples containing other herpesviruses (i.e., human cytomegalovirus [HCMV] and Epstein-Barr virus [EBV]), and two HHV-6-negative samples. Panel samples were prepared in human plasma, heat inactivated, and lyophilized. Panel distribution, data management, and analysis were coordinated by Quality Control for Molecular Diagnostics (QCMD), Glasgow, United Kingdom. Fifty-one laboratories participated and submitted 57 data sets. Eleven (19.3%) data sets were generated using conventional in-house assays, 11 (19.3%) data sets using commercial real-time PCR assays, and 35 (61.4%) data sets using in-house real-time PCR assays. The presence of HHV-6 DNA at viral loads exceeding 6,000 copies/ml was detected by all participants, and over 80% of the participants still reported correct qualitative results for the sample containing just over 200 copies/ml. The false-positivity rate was 1.8% for both the negative samples and the samples containing HCMV or EBV DNA. The majority (23/33; 69.7%) of quantitative data sets were generated using in-house real-time PCR assays. The standard deviations of the geometric means of the samples ranged from 0.5 to 0.7 log(10). The results of this first international EQA demonstrate encouraging analytical sensitivity for the detection of HHV-6-DNA in human plasma, although we observed extensive interlaboratory variation of quantitative HHV-6 DNA results. Standardization needs to be improved to allow further elucidation of the clinical significance of HHV-6 loads.
Figures
Similar articles
-
Quantification of two viral transcripts by real time PCR to investigate human herpesvirus type 6 active infection.J Clin Virol. 2014 Feb;59(2):94-9. doi: 10.1016/j.jcv.2013.11.014. Epub 2013 Dec 7. J Clin Virol. 2014. PMID: 24380721
-
Comparative evaluation of a laboratory developed real-time PCR assay and the RealStar® HHV-6 PCR Kit for quantitative detection of human herpesvirus 6.J Virol Methods. 2017 Aug;246:112-116. doi: 10.1016/j.jviromet.2017.05.001. Epub 2017 May 2. J Virol Methods. 2017. PMID: 28476346
-
Development of a new quantitative real-time HHV-6-PCR and monitoring of HHV-6 DNAaemia after liver transplantation.J Virol Methods. 2012 Apr;181(1):25-36. doi: 10.1016/j.jviromet.2012.01.007. Epub 2012 Jan 24. J Virol Methods. 2012. PMID: 22301197
-
Molecular diagnostic tests for human herpesvirus 6.Pediatr Infect Dis J. 2011 Jul;30(7):604-5. doi: 10.1097/INF.0b013e318224947f. Pediatr Infect Dis J. 2011. PMID: 21673548 Review. No abstract available.
-
[Diagnostic tests: Human herpesvirus-6 (HHV-6) and human herpesvirus-7 (HHV-7)].Nihon Rinsho. 2005 Jul;63 Suppl 7:307-9. Nihon Rinsho. 2005. PMID: 16111258 Review. Japanese. No abstract available.
Cited by
-
External quality assessment for enterovirus 71 and coxsackievirus A16 detection by reverse transcription-PCR using armored RNA as a virus surrogate.J Clin Microbiol. 2011 Oct;49(10):3591-5. doi: 10.1128/JCM.00686-11. Epub 2011 Aug 24. J Clin Microbiol. 2011. PMID: 21865426 Free PMC article.
-
Human herpesvirus 6 in transplant recipients: an update on diagnostic and treatment strategies.Curr Opin Infect Dis. 2019 Dec;32(6):584-590. doi: 10.1097/QCO.0000000000000592. Curr Opin Infect Dis. 2019. PMID: 31567413 Free PMC article. Review.
-
Evaluation of an automated system for the quantitation of human Herpesvirus-6 DNA from clinical specimens.Pract Lab Med. 2023 Aug 5;36:e00329. doi: 10.1016/j.plabm.2023.e00329. eCollection 2023 Aug. Pract Lab Med. 2023. PMID: 37649537 Free PMC article.
-
Human herpesvirus-6, HHV-8 and parvovirus B19 after allogeneic hematopoietic cell transplant: the lesser-known viral complications.Curr Opin Infect Dis. 2024 Aug 1;37(4):245-253. doi: 10.1097/QCO.0000000000001020. Epub 2024 May 6. Curr Opin Infect Dis. 2024. PMID: 38726832 Free PMC article. Review.
-
Performance evaluation of the SMG HHV-6 Q Real-Time PCR Kit for quantitative detection and differentiation of human herpesvirus 6A and 6B.Microbiol Spectr. 2024 Apr 2;12(4):e0424923. doi: 10.1128/spectrum.04249-23. Epub 2024 Mar 7. Microbiol Spectr. 2024. PMID: 38451228 Free PMC article.
References
-
- de Pagter, A. P. J., R. Schuurman, H. Visscher, M. de Vos, M. Bierings, A. M. van Loon, C. S. Uiterwaal, D. van Baarle, E. A. Sanders, and J. Boelens. 2008. Human herpes virus 6 plasma DNA positivity after hematopoietic stem cell transplantation in children: an important risk factor for clinical outcome. Biol. Blood Marrow Transplant. 14:831-839. - PubMed
-
- Drobyski, W. R., M. Eberle, D. Majewski, and L. A. Baxter-Lowe. 1993. Prevalence of human herpesvirus 6 variant A and B infections in bone marrow transplant recipients as determined by polymerase chain reaction and sequence-specific oligonucleotide probe hybridization. J. Clin. Microbiol. 31:1515-1520. - PMC - PubMed
-
- Hall, C. B., M. T. Caserta, K. C. Schnabel, C. Long, L. G. Epstein, R. A. Insel, and S. Dewhurst. 1998. Persistence of human herpesvirus 6 according to site and variant: possible greater neurotropism of variant A. Clin. Infect. Dis. 26:132-137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

