Odors activate dual pathways, a TRPC2 and a AA-dependent pathway, in mouse vomeronasal neurons
- PMID: 20147653
- PMCID: PMC2867386
- DOI: 10.1152/ajpcell.00271.2009
Odors activate dual pathways, a TRPC2 and a AA-dependent pathway, in mouse vomeronasal neurons
Abstract
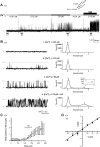
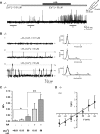
Located at the anterior portion of the nose, the paired vomeronasal organs (VNO) detect odors and pheromones. In vomeronasal sensory neurons (VSNs) odor responses are mainly mediated by phospholipase C (PLC), stimulation of which elevates diacylglycerol (DAG). DAG activates a transient receptor potential channel (TRPC2) leading to cell depolarization. In this study, we used a natural stimulus, urine, to elicit odor responses in VSNs and found urine responses persisted in TRPC2(-/-) mice, suggesting the existence of a TRPC2-independent signal transduction pathway. Using perforated patch-clamp recordings on isolated VSNs from wild-type (WT) and TRPC2(-/-) mice, we found a PLC inhibitor blocked urine responses from all VSNs. Furthermore, urine responses were reduced by blocking DAG lipase, an enzyme that produces arachidonic acid (AA), in WT mice and abolished in TRPC2(-/-) mice. Consistently, direct stimulation with AA activated an inward current that was independent of TRPC2 channels but required bath Ca(2+) and was blocked by Cd(2+). With the use of inside-out patches from TRPC2(-/-) VSNs, we show that AA activated a channel that also required Ca(2+). Together, these data from WT and TRPC2(-/-) mice suggest that both DAG and its metabolite, AA, mediate excitatory odor responses in VSNs, by activating two types of channels, a TRPC2 and a separate Ca(2+)-permeable channel.
Figures

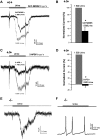
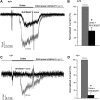
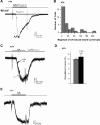
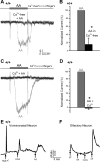
Similar articles
-
Urine stimulation activates BK channels in mouse vomeronasal neurons.J Neurophysiol. 2008 Oct;100(4):1824-34. doi: 10.1152/jn.90555.2008. Epub 2008 Aug 13. J Neurophysiol. 2008. PMID: 18701755 Free PMC article.
-
Vomeronasal sensory neurons from Sternotherus odoratus (stinkpot/musk turtle) respond to chemosignals via the phospholipase C system.J Exp Biol. 2006 May;209(Pt 10):1914-27. doi: 10.1242/jeb.02206. J Exp Biol. 2006. PMID: 16651557 Free PMC article.
-
A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction.Neuron. 2003 Oct 30;40(3):551-61. doi: 10.1016/s0896-6273(03)00675-5. Neuron. 2003. PMID: 14642279
-
The TRPC2 ion channel and pheromone sensing in the accessory olfactory system.Naunyn Schmiedebergs Arch Pharmacol. 2005 Apr;371(4):245-50. doi: 10.1007/s00210-005-1028-8. Naunyn Schmiedebergs Arch Pharmacol. 2005. PMID: 15871013 Review.
-
Neurobiology of TRPC2: from gene to behavior.Pflugers Arch. 2005 Oct;451(1):61-71. doi: 10.1007/s00424-005-1432-4. Epub 2005 Jun 22. Pflugers Arch. 2005. PMID: 15971083 Review.
Cited by
-
The genomic basis of vomeronasal-mediated behaviour.Mamm Genome. 2014 Feb;25(1-2):75-86. doi: 10.1007/s00335-013-9463-1. Epub 2013 Jul 25. Mamm Genome. 2014. PMID: 23884334 Free PMC article. Review.
-
TRICK or TRP? What Trpc2(-/-) mice tell us about vomeronasal organ mediated innate behaviors.Front Neurosci. 2015 Jun 23;9:221. doi: 10.3389/fnins.2015.00221. eCollection 2015. Front Neurosci. 2015. PMID: 26157356 Free PMC article.
-
Noradrenaline modulates sensory information in mouse vomeronasal sensory neurons.iScience. 2024 Sep 2;27(10):110872. doi: 10.1016/j.isci.2024.110872. eCollection 2024 Oct 18. iScience. 2024. PMID: 39328934 Free PMC article.
-
Paradoxical contribution of SK3 and GIRK channels to the activation of mouse vomeronasal organ.Nat Neurosci. 2012 Sep;15(9):1236-44. doi: 10.1038/nn.3173. Epub 2012 Jul 29. Nat Neurosci. 2012. PMID: 22842147 Free PMC article.
-
Phospholipase C and diacylglycerol mediate olfactory responses to amino acids in the main olfactory epithelium of an amphibian.PLoS One. 2014 Jan 28;9(1):e87721. doi: 10.1371/journal.pone.0087721. eCollection 2014. PLoS One. 2014. PMID: 24489954 Free PMC article.
References
-
- Bae YS, Lee TG, Park JC, Hur JH, Kim Y, Heo K, Kwak JY, Suh PG, Ryu SH. Identification of a compound that directly stimulates phospholipase C activity. Mol Pharmacol 63: 1043–1050, 2003 - PubMed
-
- Baxi KN, Dorries KM, Eisthen HL. Is the vomeronasal system really specialized for detecting pheromones? Trends Neurosci 29: 1–7, 2006 - PubMed
-
- Bigiani A, Mucignat-Caretta C, Montani G, Tirindelli R. Pheromone reception in mammals. Rev Physiol Biochem Pharmacol 154: 1–35, 2005 - PubMed
-
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 16: 1145–1150, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

