Comparison of the liquid-ordered bilayer phases containing cholesterol or 7-dehydrocholesterol in modeling Smith-Lemli-Opitz syndrome
- PMID: 20147702
- PMCID: PMC2882720
- DOI: 10.1194/jlr.M003467
Comparison of the liquid-ordered bilayer phases containing cholesterol or 7-dehydrocholesterol in modeling Smith-Lemli-Opitz syndrome
Abstract
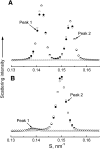
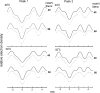
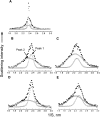
The phase behavior of egg sphingomyelin (ESM) mixtures with cholesterol or 7-dehydrocholesterol (7-DHC) has been investigated by independent methods: fluorescence microscopy, X-ray diffraction, and electron spin resonance spectroscopy. In giant vesicles, cholesterol-enriched domains appeared as large and clearly delineated domains assigned to a liquid-ordered (Lo) phase. The domains containing 7-DHC were smaller and had more diffuse boundaries. Separation of a gel phase assigned by X-ray examination to pure sphingomyelin domains coexisting with sterol-enriched domains was observed at temperatures less than 38 degrees C in binary mixtures containing 10-mol% sterol. At higher sterol concentrations, the coexistence of liquid-ordered and liquid-disordered phases was evidenced in the temperature range 20 degrees -50 degrees C. Calculated electron density profiles indicated the location of 7-DHC was more loosely defined than cholesterol, which is localized precisely at a particular depth along the bilayer normal. ESR spectra of spin-labeled fatty acid partitioned in the liquid-ordered component showed a similar, high degree of order for both sterols in the center of the bilayer, but it was higher in the coexisting disordered phase for 7-DHC. The differences detected in the models of the lipid membrane matrix are said to initiate the deleterious consequences of the Smith-Lemli-Opitz syndrome.
Figures





Similar articles
-
Compared effects of cholesterol and 7-dehydrocholesterol on sphingomyelin-glycerophospholipid bilayers studied by ESR.Biophys Chem. 2000 May 15;84(3):269-79. doi: 10.1016/s0301-4622(00)00135-6. Biophys Chem. 2000. PMID: 10852314
-
Building up of the liquid-ordered phase formed by sphingomyelin and cholesterol.Biophys J. 2005 Jun;88(6):4032-44. doi: 10.1529/biophysj.104.054155. Epub 2005 Mar 11. Biophys J. 2005. PMID: 15764672 Free PMC article.
-
Interaction of cholesterol with sphingomyelin in mixed membranes containing phosphatidylcholine, studied by spin-label ESR and IR spectroscopies. A possible stabilization of gel-phase sphingolipid domains by cholesterol.Biochemistry. 2001 Feb 27;40(8):2614-22. doi: 10.1021/bi0019803. Biochemistry. 2001. PMID: 11327885
-
[Inborn error of cholesterol biosynthesis: Smith-Lemli-Opitz syndrome].Orv Hetil. 2015 Oct 18;156(42):1695-702. doi: 10.1556/650.2015.30256. Orv Hetil. 2015. PMID: 26551309 Review. Hungarian.
-
Fluorescence methods to detect phase boundaries in lipid bilayer mixtures.Biochim Biophys Acta. 2005 Dec 30;1746(3):186-92. doi: 10.1016/j.bbamcr.2005.05.008. Epub 2005 Jun 15. Biochim Biophys Acta. 2005. PMID: 15992943 Free PMC article. Review.
Cited by
-
Smith-Lemli-Opitz syndrome: A pathophysiological manifestation of the Bloch hypothesis.Front Mol Biosci. 2023 Jan 12;10:1120373. doi: 10.3389/fmolb.2023.1120373. eCollection 2023. Front Mol Biosci. 2023. PMID: 36714259 Free PMC article. Review.
-
Brain Cholesterol Metabolism and Its Defects: Linkage to Neurodegenerative Diseases and Synaptic Dysfunction.Acta Naturae. 2016 Jan-Mar;8(1):58-73. Acta Naturae. 2016. PMID: 27099785 Free PMC article.
-
Structural dissection of ergosterol metabolism reveals a pathway optimized for membrane phase separation.Sci Adv. 2025 Apr 25;11(17):eadu7190. doi: 10.1126/sciadv.adu7190. Epub 2025 Apr 23. Sci Adv. 2025. PMID: 40267201 Free PMC article.
-
Impact of sterol tilt on membrane bending rigidity in cholesterol and 7DHC-containing DMPC membranes.Soft Matter. 2011 Nov 7;7(21):10299-10312. doi: 10.1039/C1SM05937H. Soft Matter. 2011. PMID: 23173009 Free PMC article.
-
Undulations Drive Domain Registration from the Two Membrane Leaflets.Biophys J. 2017 Jan 24;112(2):339-345. doi: 10.1016/j.bpj.2016.12.023. Biophys J. 2017. PMID: 28122219 Free PMC article.
References
-
- Smith D. W., Lemli L., Opitz J. M. 1964. Newly recognised syndrome of multiple congenital anomalies. J. Pediatr. 64: 210–217. - PubMed
-
- Waterham H. R. 2006. Defects of cholesterol biosynthesis. FEBS Lett. 580: 5442–5449. - PubMed
-
- Witsch-Baumgartner M. 2008. DHCR7 mutations causing the Smith-Lemli-Opitz syndrome. Future Lipidol. 3: 585–593.
-
- Chevy F., Humbert L., Wolf C. 2005. Sterol profiling of amniotic fluid: a routine method for the detection of distal cholesterol synthesis deficit. Prenat. Diagn. 25: 1000–1006. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

