Developmental programming: effect of prenatal steroid excess on intraovarian components of insulin signaling pathway and related proteins in sheep
- PMID: 20147730
- PMCID: PMC2874494
- DOI: 10.1095/biolreprod.109.082719
Developmental programming: effect of prenatal steroid excess on intraovarian components of insulin signaling pathway and related proteins in sheep
Abstract
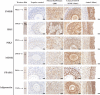
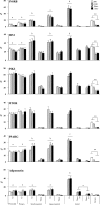
Prenatal testosterone (T) excess increases ovarian follicular recruitment, follicular persistence, insulin resistance, and compensatory hyperinsulinemia. Considering the importance of insulin in ovarian physiology, in this study, using prenatal T- and dihydrotestosterone (DHT, a nonaromatizable androgen)-treated female sheep, we tested the hypothesis that prenatal androgen excess alters the intraovarian insulin signaling cascade and metabolic mediators that have an impact on insulin signaling. Changes in ovarian insulin receptor (INSRB), insulin receptor substrate 1 (IRS1), mammalian target of rapamycin (MTOR), phosphatidylinositol 3-kinase (PIK3), peroxisome proliferator-activated receptor-gamma (PPARG), and adiponectin proteins were determined at fetal (Days 90 and 140), postpubertal (10 mo), and adult (21 mo) ages by immunohistochemistry. Results indicated that these proteins were expressed in granulosa, theca, and stromal compartments, with INSRB, IRS1, PPARG, and adiponectin increasing in parallel with advanced follicular differentiation. Importantly, prenatal T excess induced age-specific changes in PPARG and adiponectin expression, with increased PPARG expression evident during fetal life and decreased antral follicular adiponectin expression during adult life. Comparison of developmental changes in prenatal T and DHT-treated females found that the effects on PPARG were programmed by androgenic actions of T, whereas the effects on adiponectin were likely by its estrogenic action. These results suggest a role for PPARG in the programming of ovarian disruptions by prenatal T excess, including a decrease in antral follicular adiponectin expression and a contributory role for adiponectin in follicular persistence and ovulatory failure.
Figures

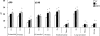
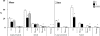
Similar articles
-
Developmental Programming: Prenatal Testosterone Excess on Ovarian SF1/DAX1/FOXO3.Reprod Sci. 2020 Jan;27(1):342-354. doi: 10.1007/s43032-019-00029-0. Epub 2020 Jan 1. Reprod Sci. 2020. PMID: 32046386 Free PMC article.
-
Developmental programming: differential effects of prenatal testosterone excess on insulin target tissues.Endocrinology. 2010 Nov;151(11):5165-73. doi: 10.1210/en.2010-0666. Epub 2010 Sep 15. Endocrinology. 2010. PMID: 20843997 Free PMC article.
-
Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology in sheep.Biol Reprod. 2009 Apr;80(4):726-36. doi: 10.1095/biolreprod.108.072801. Epub 2008 Dec 17. Biol Reprod. 2009. PMID: 19092114 Free PMC article.
-
Reproduction Symposium: developmental programming of reproductive and metabolic health.J Anim Sci. 2014 Aug;92(8):3199-210. doi: 10.2527/jas.2014-7637. J Anim Sci. 2014. PMID: 25074449 Free PMC article. Review.
-
Steroidogenic versus Metabolic Programming of Reproductive Neuroendocrine, Ovarian and Metabolic Dysfunctions.Neuroendocrinology. 2015;102(3):226-37. doi: 10.1159/000381830. Epub 2015 Apr 1. Neuroendocrinology. 2015. PMID: 25832114 Free PMC article. Review.
Cited by
-
Sheep models of polycystic ovary syndrome phenotype.Mol Cell Endocrinol. 2013 Jul 5;373(1-2):8-20. doi: 10.1016/j.mce.2012.10.005. Epub 2012 Oct 16. Mol Cell Endocrinol. 2013. PMID: 23084976 Free PMC article. Review.
-
Developmental Programming: Prenatal Testosterone Excess on Ovarian SF1/DAX1/FOXO3.Reprod Sci. 2020 Jan;27(1):342-354. doi: 10.1007/s43032-019-00029-0. Epub 2020 Jan 1. Reprod Sci. 2020. PMID: 32046386 Free PMC article.
-
Adiponectin Expression in the Porcine Ovary during the Oestrous Cycle and Its Effect on Ovarian Steroidogenesis.Int J Endocrinol. 2014;2014:957076. doi: 10.1155/2014/957076. Epub 2014 Mar 26. Int J Endocrinol. 2014. PMID: 24790602 Free PMC article.
-
Stimulatory effect of insulin on theca-interstitial cell proliferation and cell cycle regulatory proteins through MTORC1 dependent pathway.Mol Cell Endocrinol. 2013 Feb 5;366(1):81-9. doi: 10.1016/j.mce.2012.12.004. Epub 2012 Dec 19. Mol Cell Endocrinol. 2013. PMID: 23261705 Free PMC article.
-
Developmental Programming: Does Prenatal Steroid Excess Disrupt the Ovarian VEGF System in Sheep?Biol Reprod. 2015 Sep;93(3):58. doi: 10.1095/biolreprod.115.131607. Epub 2015 Jul 15. Biol Reprod. 2015. PMID: 26178718 Free PMC article.
References
-
- Webb R, Campbell BK.Development of the dominant follicle: mechanisms of selection and maintenance of oocyte quality. Soc Reprod Fertil Suppl 2007; 64: 141–163. - PubMed
-
- Padmanabhan V, Manikkam M, Recabarren S, Foster D.Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol 2006; 246: 165–174. - PubMed
-
- Dunaif A.Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997; 18: 774–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

