The bone marrow-derived human mesenchymal stem cell: potential progenitor of the endometrial stromal fibroblast
- PMID: 20147733
- PMCID: PMC2874495
- DOI: 10.1095/biolreprod.109.082867
The bone marrow-derived human mesenchymal stem cell: potential progenitor of the endometrial stromal fibroblast
Erratum in
- Biol Reprod. 2015 May;92(5):126
Abstract
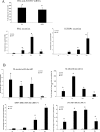
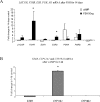
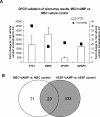
The cellular sources that contribute to the renewal of human endometrium are largely unknown. It has been suggested that endometrial stem cells originate from bone marrow-derived mesenchymal stem cells (MSC), with subsequent development into endometrial stromal fibroblasts (hESF). We hypothesized that if bone marrow-derived MSC contribute to endometrial regeneration and are progenitors of hESF, their treatment with agents known to regulate hESF differentiation could promote their differentiation down the stromal fibroblast lineage. To this end, we treated bone marrow-derived MSC with estradiol and progesterone, bone morphogenetic protein 2 (BMP2), and activators of the protein kinase A (PKA) pathway and investigated specific markers of hESF differentiation (decidualization). Furthermore, we investigated the transcriptome of these cells in response to cAMP and compared this to the transcriptome of hESF decidualized in response to activation of the PKA pathway. The data support the idea that MSC can be differentiated down the hESF pathway, as evidenced by changes in cell shape and common expression of decidual markers and other genes important in hESF differentiation and function, and that bone marrow-derived MSC may be a source of endometrial stem/progenitor cells. In addition, we identified MSC-specific markers that distinguish them from other fibroblasts and, in particular, from hESF, which is of biologic relevance and practical value to the field of endometrial stem cell research.
Figures



References
-
- Hess A, Nayak N, Giudice LC.Oviduct and endometrium: cyclic changes in primate oviduct and endometrium. Knobil E, Neill JD.The Physiology of Reproduction, 3rd ed San Diego:Academic Press;2005: 337–381.
-
- Chan RW, Schwab KE, Gargett CE.Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod 2004; 70: 1738–1750. - PubMed
-
- Caplan AI.Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 2007; 213: 341–347. - PubMed
-
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008; 3: 301–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

