Enhancement of attentional performance by selective stimulation of alpha4beta2(*) nAChRs: underlying cholinergic mechanisms
- PMID: 20147893
- PMCID: PMC2855755
- DOI: 10.1038/npp.2010.9
Enhancement of attentional performance by selective stimulation of alpha4beta2(*) nAChRs: underlying cholinergic mechanisms
Abstract
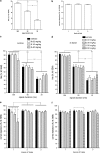
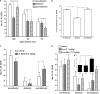
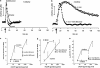
Impairments in attention are a major component of the cognitive symptoms of neuropsychiatric and neurodegenerative disorders. Using an operant sustained attention task (SAT), including a distractor condition (dSAT), we assessed the putative pro-attentional effects of the selective alpha4beta2(*) nicotinic acetylcholine receptor (nAChR) agonist S 38232 in comparison with the non-selective agonist nicotine. Neither drug benefited SAT performance. However, in interaction with the increased task demands implemented by distractor presentation, the selective agonist, but not nicotine, enhanced the detection of signals during the post-distractor recovery period. This effect is consistent with the hypothesis that second-long increases in cholinergic activity ('transients') mediate the detection of cues and that nAChR agonists augment such transients. Electrochemical recordings of prefrontal cholinergic transients evoked by S 38232 and nicotine indicated that the alpha4beta2(*) nAChR agonist evoked cholinergic transients that were characterized by a faster rise time and more rapid decay than those evoked by nicotine. Blockade of the alpha7 nAChR 'sharpens' nicotine-evoked transients; therefore, we determined the effects of co-administration of nicotine and the alpha7 nAChR antagonist methyllycaconitine on dSAT performance. Compared with vehicle and nicotine alone, this combined treatment significantly enhanced the detection of signals. These results indicate that compared with nicotine, alpha4beta2(*) nAChR agonists significantly enhance attentional performance and that the dSAT represents a useful behavioral screening tool. The combined behavioral and electrochemical evidence supports the hypothesis that nAChR agonist-evoked cholinergic transients, which are characterized by rapid rise time and fast decay, predict robust drug-induced enhancement of attentional performance.
Figures
Similar articles
-
Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling.J Neurosci. 2010 Mar 3;30(9):3518-30. doi: 10.1523/JNEUROSCI.5712-09.2010. J Neurosci. 2010. PMID: 20203212 Free PMC article.
-
Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex.J Neurosci. 2008 Apr 2;28(14):3769-80. doi: 10.1523/JNEUROSCI.5251-07.2008. J Neurosci. 2008. PMID: 18385335 Free PMC article.
-
Selective potentiation of (α4)3(β2)2 nicotinic acetylcholine receptors augments amplitudes of prefrontal acetylcholine- and nicotine-evoked glutamatergic transients in rats.Biochem Pharmacol. 2013 Nov 15;86(10):1487-96. doi: 10.1016/j.bcp.2013.09.005. Epub 2013 Sep 16. Biochem Pharmacol. 2013. PMID: 24051136 Free PMC article.
-
nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms.Biochem Pharmacol. 2009 Oct 1;78(7):658-67. doi: 10.1016/j.bcp.2009.04.019. Epub 2009 May 4. Biochem Pharmacol. 2009. PMID: 19406107 Free PMC article. Review.
-
The contribution of agonist and antagonist activities of α4β2* nAChR ligands to smoking cessation efficacy: a quantitative analysis of literature data.Psychopharmacology (Berl). 2018 Sep;235(9):2479-2505. doi: 10.1007/s00213-018-4921-9. Epub 2018 Jul 7. Psychopharmacology (Berl). 2018. PMID: 29980822 Review.
Cited by
-
Time to pay attention: attentional performance time-stamped prefrontal cholinergic activation, diurnality, and performance.J Neurosci. 2012 Aug 29;32(35):12115-28. doi: 10.1523/JNEUROSCI.2271-12.2012. J Neurosci. 2012. PMID: 22933795 Free PMC article.
-
Neural circuits for a top-down control of fear and extinction.Psychopharmacology (Berl). 2019 Jan;236(1):313-320. doi: 10.1007/s00213-018-5033-2. Epub 2018 Sep 13. Psychopharmacology (Berl). 2019. PMID: 30215217 Review.
-
Deficits in attentional control: cholinergic mechanisms and circuitry-based treatment approaches.Behav Neurosci. 2011 Dec;125(6):825-35. doi: 10.1037/a0026227. Behav Neurosci. 2011. PMID: 22122146 Free PMC article. Review.
-
Effects of nicotinic acetylcholine receptor agonists on cognition in rhesus monkeys with a chronic cocaine self-administration history.Neuropharmacology. 2013 Jan;64:479-88. doi: 10.1016/j.neuropharm.2012.08.004. Epub 2012 Aug 23. Neuropharmacology. 2013. PMID: 22921923 Free PMC article.
-
Cholinergic genetics of visual attention: Human and mouse choline transporter capacity variants influence distractibility.J Physiol Paris. 2016 Sep;110(1-2):10-18. doi: 10.1016/j.jphysparis.2016.07.001. Epub 2016 Jul 9. J Physiol Paris. 2016. PMID: 27404793 Free PMC article. Review.
References
-
- Bizarro L, Stolerman IP. Attentional effects of nicotine and amphetamine in rats at different levels of motivation. Psychopharmacology (Berl) 2003;170:271–277. - PubMed
-
- Blondel A, Sanger DJ, Moser PC. Characterization of the effects of nicotine in the five-choice serial reaction time task in rats: antagonist studies. Psychopharmacology (Berl) 2000;149:293–305. - PubMed
-
- Bushnell PJ, Oshiro WM, Padnos BK. Detection of visual signals by rats: effects of chlordiazepoxide and cholinergic and adrenergic drugs on sustained attention. Psychopharmacology (Berl) 1997;134:230–241. - PubMed
-
- Dunbar GC, Inglis F, Kuchibhatla R, Sharma T, Tomlinson M, Wamsley J. Effect of ispronicline, a neuronal nicotinic acetylcholine receptor partial agonist, in subjects with age associated memory impairment (AAMI) J Psychopharmacol. 2007;21:171–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

