Recombinant Activated Factor VII (rFVIIa) in the Management of Major Obstetric Haemorrhage: A Case Series and a Proposed Guideline for Use
- PMID: 20148069
- PMCID: PMC2817503
- DOI: 10.1155/2009/364843
Recombinant Activated Factor VII (rFVIIa) in the Management of Major Obstetric Haemorrhage: A Case Series and a Proposed Guideline for Use
Abstract
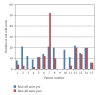
Major obstetric haemorrhage remains a significant cause of maternal morbidity and mortality. Previous case reports suggest the potential benefit of recombinant activated factor VII (rFVIIa: NovoSeven(R)) as a haemostatic agent. We performed a retrospective review of the use of rVIIa in major obstetric haemorrhage in the Northern Region between July 2004 and February 2007. Fifteen women received rFVIIa. The median patient age was 34 years. Major haemorrhage occurred antepartum (5 patients), intrapartum (1), and postpartum (9). All women received an initial dose of 90 mcg/kg rFVIIa and one received 2 further doses. Bleeding stopped or decreased in 12 patients (80%). Additional measures included antifibrinolytic and uterotonic agents, Rusch balloon insertion, uterine curettage/packing, and vessel embolisation. Eight patients required hysterectomy. All women survived to discharge from hospital. No adverse events, including thrombosis, were recorded. This study provides further support for the safety and efficacy of rFVIIa as adjunct therapy in major obstetric haemorrhage.
Figures
References
-
- Why mothers die. A report on confidential enquiries into maternal deaths in the UK 2000–2002. The Department of Health. Confidential Enquiry into maternal and child health.
-
- Dutton RP, McCunn M, Hyder M, et al. Factor VIIa for correction of traumatic coagulopathy. Journal of Trauma. 2004;57(4):709–719. - PubMed
-
- Mittal S, Watson HG. A critical appraisal of the use of recombinant factor VIIa in acquired bleeding conditions. British Journal of Haematology. 2006;133(4):355–363. - PubMed
-
- Mayo A, Misgav M, Kluger Y, et al. Recombinant activated factor VII (NovoSevenTM): addition to replacement therapy in acute, uncontrolled and life-threatening bleeding. Vox Sanguinis. 2004;87(1):34–40. - PubMed
-
- O’Connell NM, Perry DJ, Hodgson AJ, O’Shaughnessy DF, Laffan MA, Smith OP. Recombinant FVIIa in the management of uncontrolled hemorrhage. Transfusion. 2003;43(12):1711–1716. - PubMed
LinkOut - more resources
Full Text Sources