Sensitive electrochemical detection of horseradish peroxidase at disposable screen-printed carbon electrode
- PMID: 20148182
- PMCID: PMC2817974
- DOI: 10.1002/elan.200804287
Sensitive electrochemical detection of horseradish peroxidase at disposable screen-printed carbon electrode
Abstract
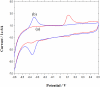
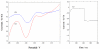
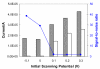
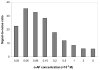
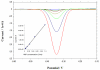
A rapid, simple and sensitive electrochemical assay of horseradish peroxidase (HRP) performed on disposable screen-printed carbon electrode was developed. HRP activities were monitored by square-wave voltammetric (SWV) measuring the electroactive enzymatic product in the presence of o-aminophenol and hydrogen peroxide substrate solution. SWV analysis demonstrated a greater sensitivity and shorter analysis time than the widely used amperometric and differential-pulsed voltammetric methods. The voltammetric characteristics of substrate and enzymatic product as well as the parameters of SWV analysis were optimized. Under optimized conditions, a linear response for HRP from 0.003 - 0.1 U/mL and a detection limit of 0.002 U/mL (1.25×10(-15) mol in 25 μL) were obtained with a good precision (RSD = 8%; n = 6). This rapid and sensitive HRP assay with microliters-assay volume could be readily integrated to portable devices and point-of-care (POC) diagnosis applications.
Figures
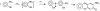
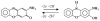
References
-
- Seeger TF, Bartlett B, Coskran TM, Culp JS, James LC, Krull DL, Lanfear J, Ryan AM, Schmidt CJ, Strick CA. Brain Res. 2003;985:113. - PubMed
-
- Lin J, Yan F, Hu X, Ju H. J. Immunol. Meth. 2004;291:165. - PubMed
-
- Barroso-Chinea P, Aymerich MS, Castle MM, Perez-Manso M, Tunon T, Erro E, Lanciego JL. J. Neurosci. Meth. 2007;162:119. - PubMed
-
- Uhl J, Newton RC. J. Immunol. Meth. 1988;110:79. - PubMed
-
- Salinas E, Torriero AAJ, Battaglini F, Sanz MI, Olsina R, Raba J. Biosens. Bioelectron. 2005;21:313. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
