Enantioselective conjugate silyl additions to cyclic and acyclic unsaturated carbonyls catalyzed by Cu complexes of chiral N-heterocyclic carbenes
- PMID: 20148561
- PMCID: PMC2836128
- DOI: 10.1021/ja910989n
Enantioselective conjugate silyl additions to cyclic and acyclic unsaturated carbonyls catalyzed by Cu complexes of chiral N-heterocyclic carbenes
Abstract
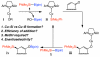
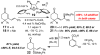
An efficient Cu-catalyzed protocol for enantioselective addition of a dimethylphenylsilanyl group to a wide range of cyclic and acyclic unsaturated ketones, esters, acrylonitriles, and alpha,beta,gamma,delta-dienones is disclosed. Reactions are performed in the presence of 1-2 mol % of commercially available and inexpensive CuCl, a readily accessible monodentate imidazolinium salt, and commercially available (dimethylphenylsilyl)pinacolatoboron. Cu-catalyzed enantioselective conjugate additions proceed to completion within only 2 h to afford the desired silanes in 87-97% yield and 90:10-99:1 enantiomeric ratio (er). Use of a proton source (e.g., MeOH) is not required; accordingly, synthetically versatile alpha-silyl boron enolates can be obtained. The special utility of the present protocol, in comparison with the related catalytic enantioselective aldol and boronate conjugate additions, is discussed and illustrated through various functionalizations of the enantiomerically enriched beta-silylcarbonyls.
Figures
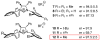
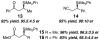

Similar articles
-
Enantioselective synthesis of boron-substituted quaternary carbons by NHC-Cu-catalyzed boronate conjugate additions to unsaturated carboxylic esters, ketones, or thioesters.J Am Chem Soc. 2010 Aug 11;132(31):10630-3. doi: 10.1021/ja104777u. J Am Chem Soc. 2010. PMID: 20681680 Free PMC article.
-
Metal-free catalytic enantioselective C-B bond formation: (pinacolato)boron conjugate additions to α,β-unsaturated ketones, esters, Weinreb amides, and aldehydes promoted by chiral N-heterocyclic carbenes.J Am Chem Soc. 2012 May 16;134(19):8277-85. doi: 10.1021/ja302929d. Epub 2012 May 4. J Am Chem Soc. 2012. PMID: 22559866 Free PMC article.
-
Cu-catalyzed asymmetric conjugate additions of dialkyl- and diarylzinc reagents to acyclic beta-silyl-alpha,beta-unsaturated ketones. Synthesis of allylsilanes in high diastereo- and enantiomeric purity.Org Lett. 2007 Aug 2;9(16):3187-90. doi: 10.1021/ol071331k. Epub 2007 Jun 30. Org Lett. 2007. PMID: 17602643
-
Sulfonate N-Heterocyclic Carbene-Copper Complexes: Uniquely Effective Catalysts for Enantioselective Synthesis of C-C, C-B, C-H, and C-Si Bonds.Angew Chem Int Ed Engl. 2020 Nov 23;59(48):21304-21359. doi: 10.1002/anie.202003755. Epub 2020 Aug 26. Angew Chem Int Ed Engl. 2020. PMID: 32364640 Free PMC article. Review.
-
Asymmetric catalysis of aldol reactions with chiral lewis bases.Acc Chem Res. 2000 Jun;33(6):432-40. doi: 10.1021/ar960027g. Acc Chem Res. 2000. PMID: 10891061 Review.
Cited by
-
Synthesis of Silylated Cyclobutanone and Cyclobutene Derivatives Involving 1,4-Addition of Zinc-Based Silicon Nucleophiles.Chemistry. 2021 Nov 22;27(65):16103-16106. doi: 10.1002/chem.202102993. Epub 2021 Oct 7. Chemistry. 2021. PMID: 34490934 Free PMC article.
-
A robust, efficient, and highly enantioselective method for synthesis of homopropargyl amines.Angew Chem Int Ed Engl. 2012 Jul 2;51(27):6618-21. doi: 10.1002/anie.201202694. Epub 2012 May 23. Angew Chem Int Ed Engl. 2012. PMID: 22623437 Free PMC article. No abstract available.
-
NHC-Cu-Catalyzed Silyl Conjugate Additions to Acyclic and Cyclic Dienones and Dienoates. Efficient Site-, Diastereo- and Enantioselective Synthesis of Carbonyl-Containing Allylsilanes.Organometallics. 2012 Nov 26;31(22):7823-7826. doi: 10.1021/om300790t. Epub 2012 Sep 19. Organometallics. 2012. PMID: 23264716 Free PMC article.
-
Sodium silylsilanolate as a precursor of silylcopper species.Chem Sci. 2022 Mar 21;13(15):4334-4340. doi: 10.1039/d2sc00227b. eCollection 2022 Apr 13. Chem Sci. 2022. PMID: 35509465 Free PMC article.
-
Recent advances in Cu-catalyzed C(sp3)-Si and C(sp3)-B bond formation.Beilstein J Org Chem. 2020 Apr 15;16:691-737. doi: 10.3762/bjoc.16.67. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32362947 Free PMC article. Review.
References
-
-
For reviews on the use of organosilanes in organic synthesis, see: Chan TH, Wang D. Chem. Rev. 1992;92:995. Jones GR, Landais Y. Tetrahedron. 1996;52:7599. Fleming I, Barbero A, Walter D. Chem. Rev. 1997;97:2063. Suginome M, Ito Y. Chem. Rev. 2000;100:3221.
-
-
-
For a review on the utility of β-silylcarbonyls in synthesis, see: Fleming I. Science of Synthesis. Vol. 4. Thieme; Stuttgart, Germany: 2002. p. 927.
-
-
-
Enantiomerically enriched β-silylcarbonyls have been prepared by catalytic conjugate hydride additions to trisubstituted Si-substituted enones. See: Lipshutz BH, Tanaka N, Taft BR, Lee C.-t. Org. Lett. 2006;8:1963.
-
-
-
Enantiomerically enriched β-silylcarbonyls can be accessed by catalytic conjugate additions of alkyl or aryl groups to silyl-substituted enones. See: Shintani R, Okamoto K, Hayashi T. Org. Lett. 2005;7:4757. Balskus EP, Jacobsen EN. J. Am. Chem. Soc. 2006;128:6810. Kacprzynski MA, Kazane SA, May TL, Hoveyda AH. Org. Lett. 2007;9:3187.
-
-
-
Catalytic non-enantioselective methods that afford β-silylcarbonyls have been disclosed. For example, see: Conjugate silane additions: Lipshutz BH, Sclafani JA, Takanami T. J. Am. Chem. Soc. 1998;120:4021. Auer G, Weiner B, Oestreich M. Synthesis. 2006:2113. Conjugate disilane additions: Tamao K, Okazaki S, Kumada M. J. Organomet. Chem. 1978;146:87. Ito H, Ishizuka T, Tateiwa J.-i., Sonoda M, Hosomi A. J. Am. Chem. Soc. 1998;120:11196. Ogoshi S, Tomiyasu S, Morita M, Kurosawa H. J. Am. Chem. Soc. 2002;124:11598. Clark CT, Lake JF, Scheidt KA. J. Am. Chem. Soc. 2004;126:84.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

